Question: Two moles of helium gas undergoes a cyclic process as shown in the figure. Assuming ideal behavior of gas, the magnitude of network done
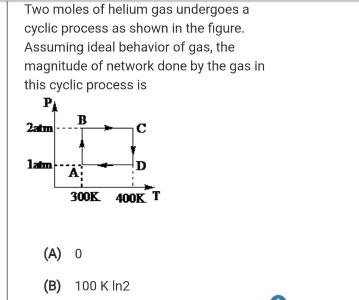
Two moles of helium gas undergoes a cyclic process as shown in the figure. Assuming ideal behavior of gas, the magnitude of network done by the gas in this cyclic process is B 2atm Jatm 300K 400K T (A) 0 (B) 100 K In2
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


