Question: please answer fast, reaction kinetics, answer all fast and i will rate An oxidation reaction of an alkene with hydrogen peroxide (H2O2) occurs in a
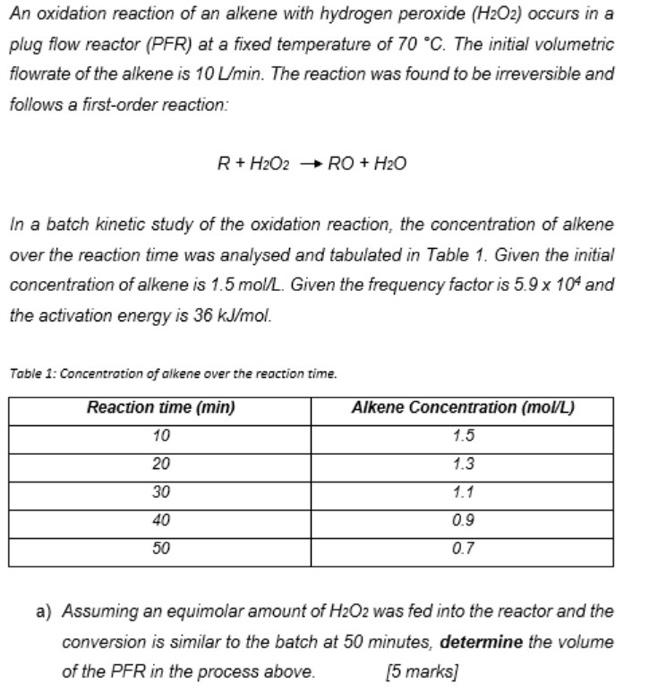
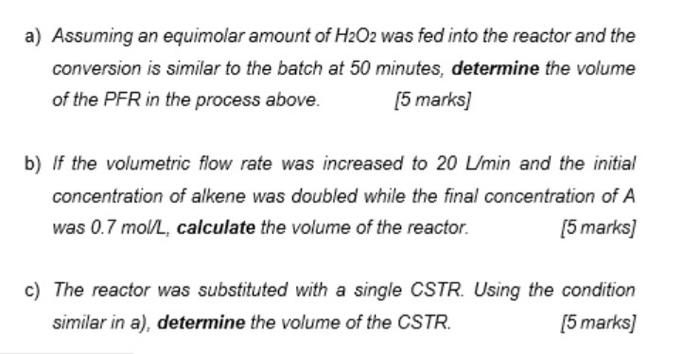
An oxidation reaction of an alkene with hydrogen peroxide (H2O2) occurs in a plug flow reactor (PFR) at a fixed temperature of 70 C. The initial volumetric flowrate of the alkene is 10 min. The reaction was found to be irreversible and follows a first-order reaction: R + H2O2 RO + H2O In a batch kinetic study of the oxidation reaction, the concentration of alkene over the reaction time was analysed and tabulated in Table 1. Given the initial concentration of alkene is 1.5 mol/L. Given the frequency factor is 5.9 x 104 and the activation energy is 36 kJ/mol. Table 1: Concentration of alkene over the reaction time. Reaction time (min) 10 Alkene Concentration (mol/L) 1.5 1.3 20 30 1.1 0.9 40 50 0.7 a) Assuming an equimolar amount of H2O2 was fed into the reactor and the conversion is similar to the batch at 50 minutes, determine the volume of the PFR in the process above. [5 marks] a) Assuming an equimolar amount of H2O2 was fed into the reactor and the conversion is similar to the batch at 50 minutes, determine the volume of the PFR in the process above. (5 marks] b) If the volumetric flow rate was increased to 20 L/min and the initial concentration of alkene was doubled while the final concentration of A was 0.7 mol/L, calculate the volume of the reactor. [5 marks) c) The reactor was substituted with a single CSTR. Using the condition similar in a), determine the volume of the CSTR. [5 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


