Question: The reactions PCI5) - PClo) + Cl) and COCl) = CO) + Clao) are simultaneously in equilibrium in an equilibrium box at constant volume.
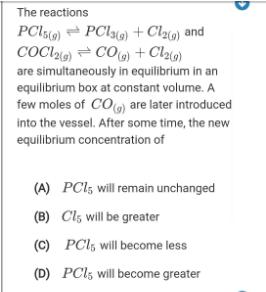
The reactions PCI5) - PClo) + Cl) and COCl) = CO) + Clao) are simultaneously in equilibrium in an equilibrium box at constant volume. A few moles of CO) are later introduced into the vessel. After some time, the new equilibrium concentration of (A) PCI; will remain unchanged (B) Cl, will be greater (C) PCl, will become less (D) PCI5 will become greater
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


