Question: Please answer for all parts MISSED THIS? Read Section 14.7. You can click on the Review link to access the section in your eText. Calculate
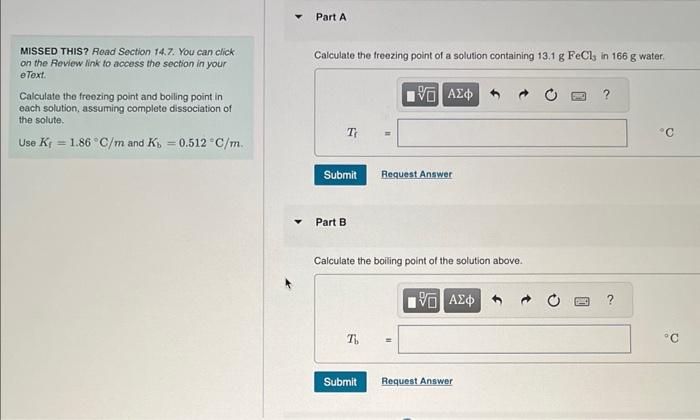
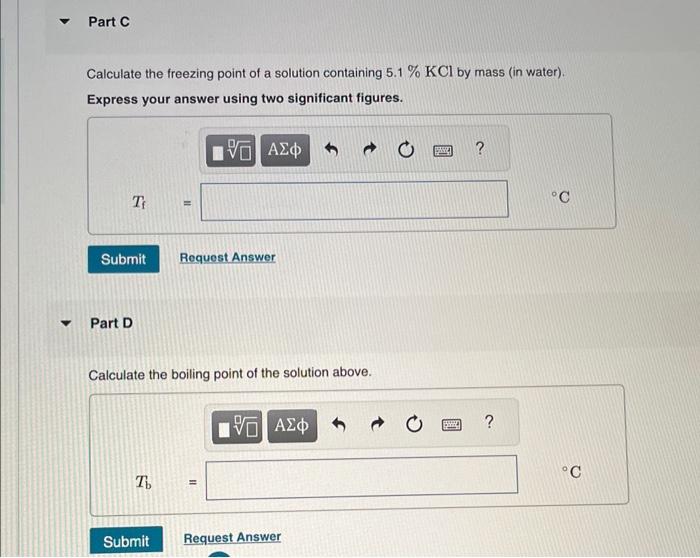
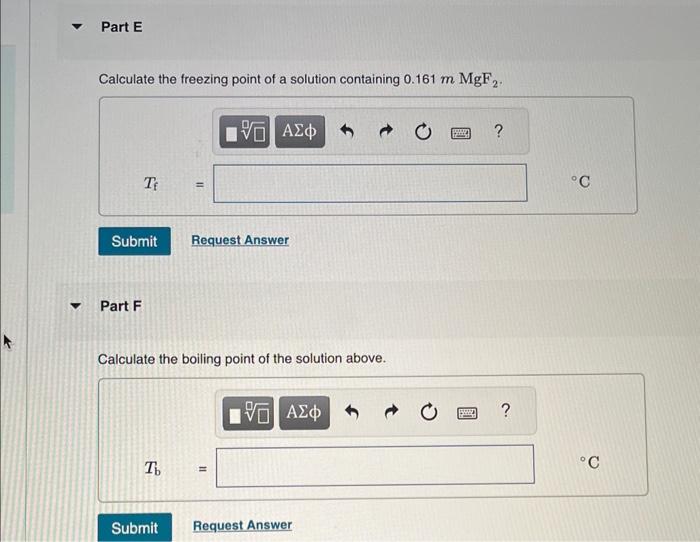
MISSED THIS? Read Section 14.7. You can click on the Review link to access the section in your eText. Calculate the freozing point and bolling point in each solution, assuming complete dissociation of the solute. Use Kf=1.86C/m and Kb=0.512C/m. Part B Calculate the bolling point of the solution above. Calculate the freezing point of a solution containing 5.1%KCl by mass (in water). Express your answer using two significant figures. Part D Calculate the boiling point of the solution above. Part F Calculate the boiling point of the solution above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


