Question: please answer fully and show all tge steps 3. Formamidine is the simplest compound containing the amidine functional group. (16 pts) NH2 a) Amidines are
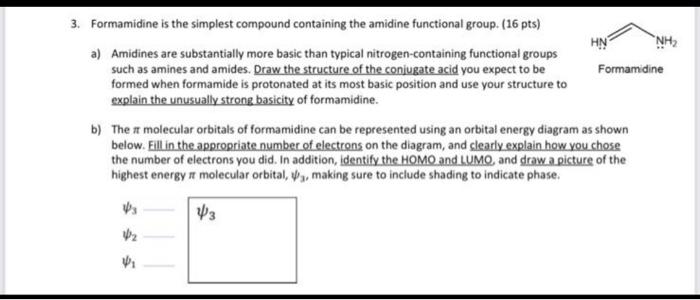
3. Formamidine is the simplest compound containing the amidine functional group. (16 pts) NH2 a) Amidines are substantially more basic than typical nitrogen-containing functional groups such as amines and amides. Draw the structure of the conjugate acid you expect to be Formamidine formed when formamide is protonated at its most basic position and use your structure to explain the unusually strong basicity of formamidine. b) The molecular orbitals of formamidine can be represented using an orbital energy diagram as shown below. Fill in the appropriate number of electrons on the diagram, and clearly explain how you chose the number of electrons you did. In addition, identify the HOMO and LUMO and draw a picture of the highest energy ft molecular orbital ,,, making sure to include shading to indicate phase. 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


