Question: Please answer fully. Thank you! 1. Draw a Newman Projection for each of the molecules below along the indicated bond with the indicated perspective. Make
Please answer fully. Thank you!
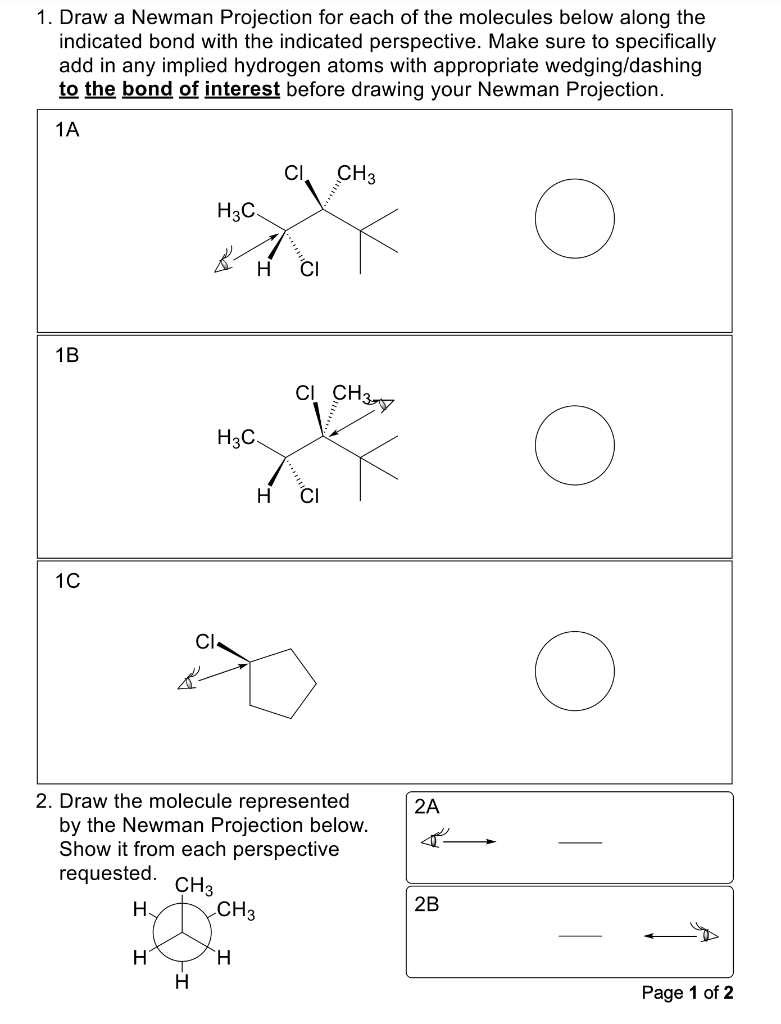
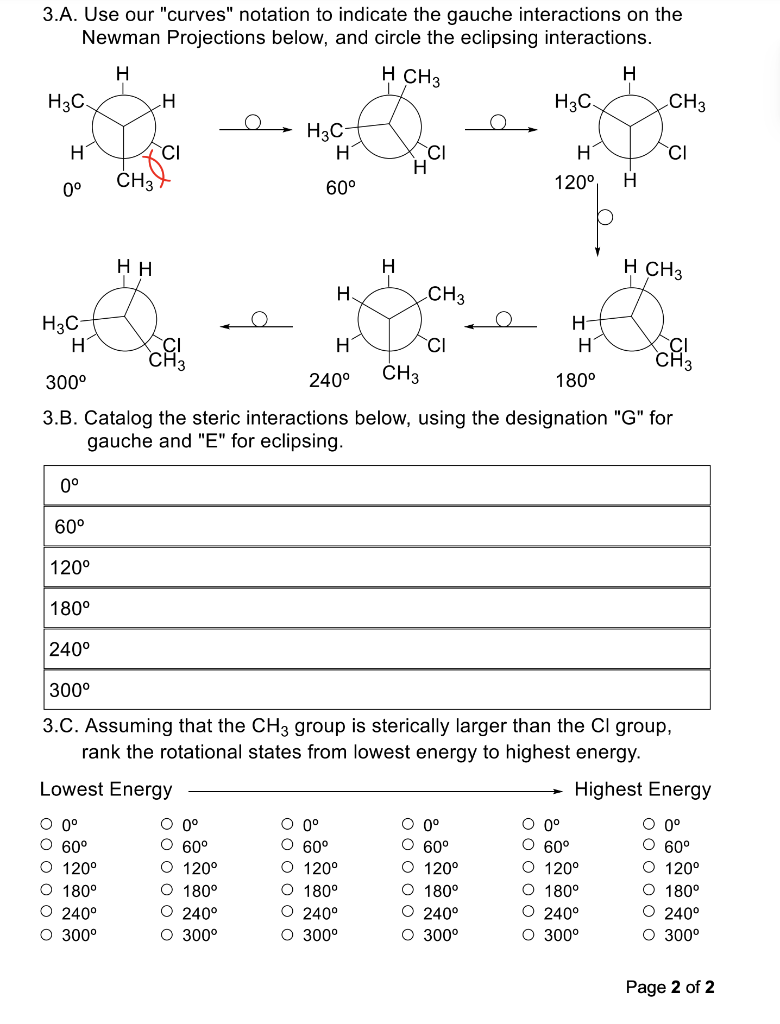
1. Draw a Newman Projection for each of the molecules below along the indicated bond with the indicated perspective. Make sure to specifically add in any implied hydrogen atoms with appropriate wedging/dashing to the bond of interest before drawing your Newman Projection. 1A 1B 1C 2. Draw the molecule represented by the Newman Projection below. Show it from each perspective requestan 2B Page 1 of 2 3.A. Use our "curves" notation to indicate the gauche interactions on the Newman Projections below, and circle the eclipsing interactions. 300 3.B. Catalog the steric interactions below, using the designation "G" for gauche and "E" for eclipsing. 3.C. Assuming that the CH3 group is sterically larger than the Cl group, rank the rotational states from lowest energy to highest energy. \begin{tabular}{clllll} Lowest Energy & & & & \multicolumn{2}{c}{ Highest Energy } \\ \cline { 2 - 6 } 0 & 0 & 0 & 0 & 0 & 0 \\ 60 & 60 & 60 & 60 & 60 & 60 \\ 120 & 120 & 120 & 120 & 120 & 120 \\ 180 & 180 & 180 & 180 & 180 & 180 \\ 240 & 240 & 240 & 240 & 240 & 240 \\ 300 & 300 & 300 & 300 & 300 & 300 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


