Question: Please answer in an understandable handwriting. An unknown sample containing mixed alkali (NaOH/NaHCO3 / Na2CO3) was analyzed using the double titration method. A 250-mg sample
Please answer in an understandable handwriting.
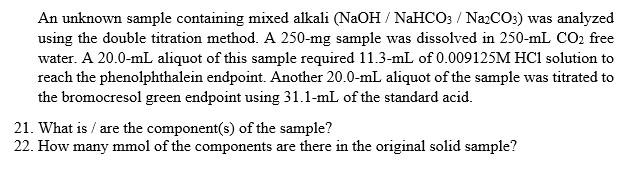
An unknown sample containing mixed alkali (NaOH/NaHCO3 / Na2CO3) was analyzed using the double titration method. A 250-mg sample was dissolved in 250-ml CO2 free water. A 20.0-ml aliquot of this sample required 11.3-mL of 0.009125M HCl solution to reach the phenolphthalein endpoint. Another 20.0-mL aliquot of the sample was titrated to the bromocresol green endpoint using 31.1-mL of the standard acid. 21. What is / are the component(s) of the sample? 22. How many mmol of the components are there in the original solid sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


