Question: Please answer it with steps ( prefer written steps not type), and if you dont know the steps i hope that give other person to
Please answer it with steps ( prefer written steps not type), and if you dont know the steps i hope that give other person to answer it. Thank you!
Subject : Thermodynamics of Materials (2)
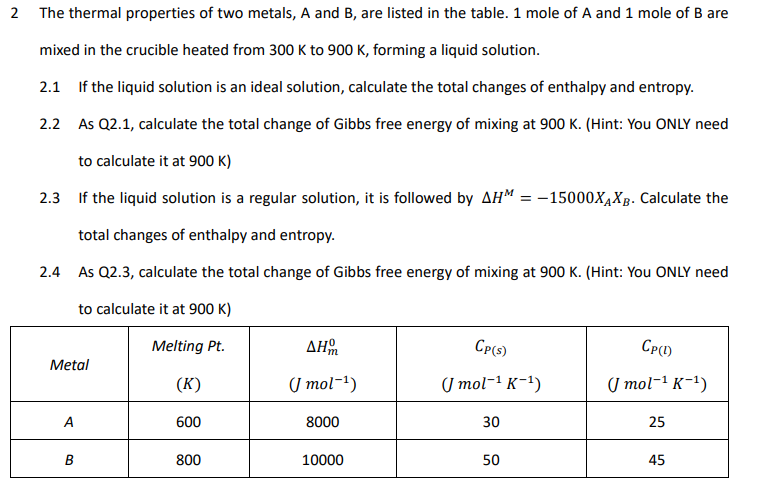
2 The thermal properties of two metals, A and B, are listed in the table. 1 mole of A and 1 mole of B are mixed in the crucible heated from 300K to 900K, forming a liquid solution. 2.1 If the liquid solution is an ideal solution, calculate the total changes of enthalpy and entropy. 2.2 As Q2.1, calculate the total change of Gibbs free energy of mixing at 900K. (Hint: You ONLY need to calculate it at 900K) 2.3 If the liquid solution is a regular solution, it is followed by HM=15000XAXB. Calculate the total changes of enthalpy and entropy. 2.4 As Q2.3, calculate the total change of Gibbs free energy of mixing at 900K. (Hint: You ONLY need
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


