Question: Please answer it within one hour, i will surely upvote your efforts 202 6. (13 points) Pseudo steady state with a heterogeneous reaction. When a
Please answer it within one hour, i will surely upvote your efforts
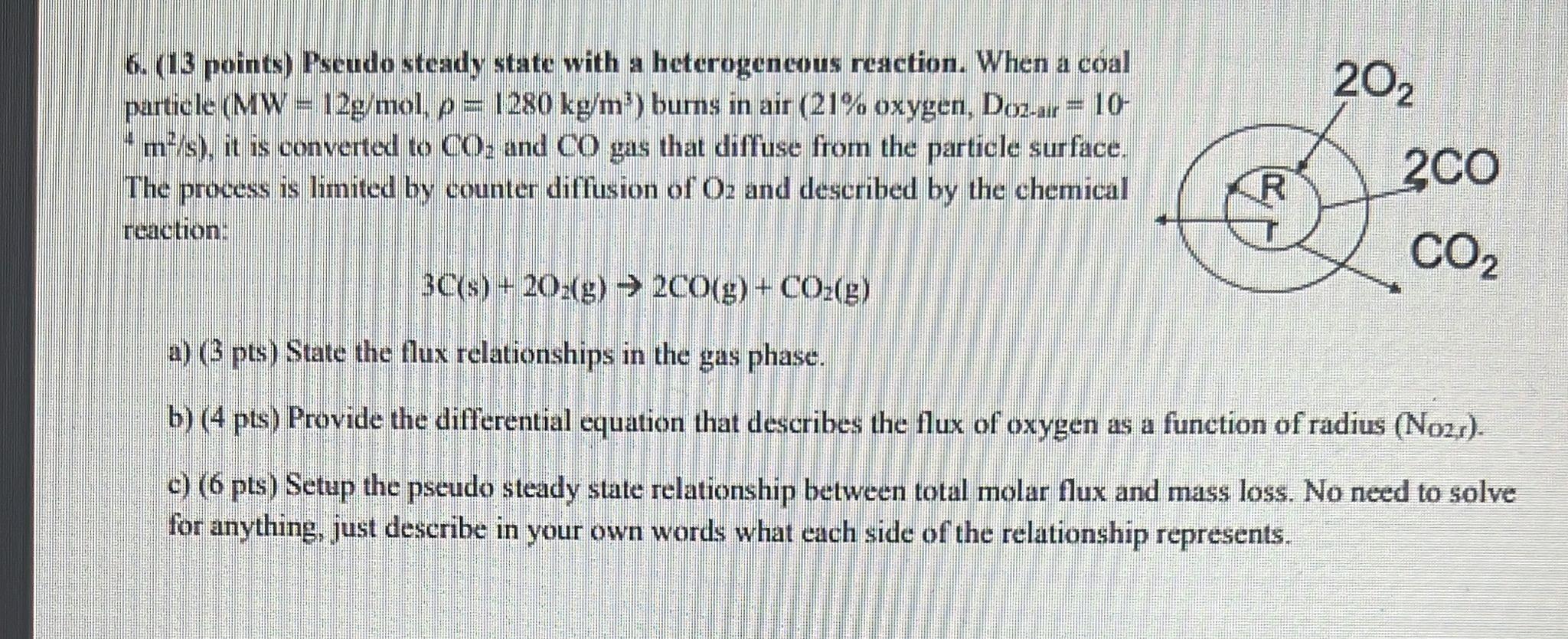
202 6. (13 points) Pseudo steady state with a heterogeneous reaction. When a coal particle (MW = 12g/mol, p= 1280 kg/m) burns in air (21% oxygen, Doz-ur = 10- 'm/s), it is converted to CO. and CO gas that diffuse from the particle surface. The process is limited by counter diffusion of O: and described by the chemical rcachon 200 CO2 3C(s) + 202(g) 200(g) + CO2(g) a) (3 pts) State the flux relationships in the gas phase. b) (4 pts) Provide the differential equation that describes the flux of oxygen as a function of radius (No21). c) (6 pts) Setup the pseudo steady state relationship between total molar flux and mass loss. No need to solve for anything, just describe in your own words what each side of the relationship represents. 202 6. (13 points) Pseudo steady state with a heterogeneous reaction. When a coal particle (MW = 12g/mol, p= 1280 kg/m) burns in air (21% oxygen, Doz-ur = 10- 'm/s), it is converted to CO. and CO gas that diffuse from the particle surface. The process is limited by counter diffusion of O: and described by the chemical rcachon 200 CO2 3C(s) + 202(g) 200(g) + CO2(g) a) (3 pts) State the flux relationships in the gas phase. b) (4 pts) Provide the differential equation that describes the flux of oxygen as a function of radius (No21). c) (6 pts) Setup the pseudo steady state relationship between total molar flux and mass loss. No need to solve for anything, just describe in your own words what each side of the relationship represents
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


