Question: please answer last 2 questions. write introduction and conclusion in detail. please write everything with detail Experiment One: Isolation of a Four-Coordinate Boron Complex Purpose
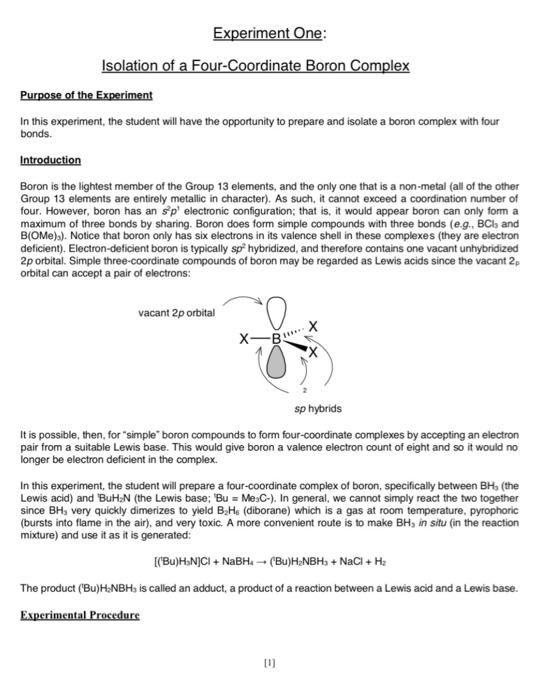

Experiment One: Isolation of a Four-Coordinate Boron Complex Purpose of the Experiment In this experiment, the student will have the opportunity to prepare and isolate a boron complex with four bonds. Introduction Boron is the lightest member of the Group 13 elements, and the only one that is a non-metal (all of the other Group 13 elements are entirely metallic in character). As such, it cannot exceed a coordination number of four. However, boron has an spl electronic configuration; that is, it would appear boron can only form a maximum of three bonds by sharing. Boron does form simple compounds with three bonds (e.g. Bch and BIOMe)s). Notice that boron only has six electrons in its valence shell in these complexes (they are electron deficient). Electron-deficient boron is typically sp hybridized, and therefore contains one vacant unhybridized 2p orbital. Simple three-coordinate compounds of boron may be regarded as Lewis acids since the vacant 2 orbital can accept a pair of electrons: vacant 2p orbital X-BX X sp hybrids It is possible, then, for "simple boron compounds to form four-coordinate complexes by accepting an electron pair from a suitable Lewis base. This would give boron a valence electron count of eight and so it would no longer be electron deficient in the complex. In this experiment, the student will prepare a four-coordinate complex of boron, specifically between BH, (the Lewis acid) and BuHN (the Lewis base; 'Bu = Me,C-). In general, we cannot simply react the two together since BH, very quickly dimerizes to yield B He (diborane) which is a gas at room temperature, pyrophoric (bursts into flame in the air), and very toxic. A more convenient route is to make BH, in situ (in the reaction mixture) and use it as it is generated: [(Bu)H.N]CI + NaBH (Bu)HENBH. + NaCl + Hz The product (Bu)H:NBH, is called an adduct, a product of a reaction between a Lewis acid and a Lewis base. Experimental Procedure [0] Special Notes and Safety Precautions The preparation of (Bu)H2NBH; should be performed in a fumehood as hydrogen gas is produced during the reaction. The preparation requires the use of tetrahydrofuran (THF), a flammable solvent so all ignition sources must be eliminated prior to using it. Also used is NaBH, which is corrosive and hygroscopic (ie, it adsorbs moisture from the atmosphere); only weigh the required portion immediately prior to its use in the reaction and make sure the container of the stock supply is tightly closed after use. Synthesis of a 'BuNH, adduct of BH: (Bu)HNBH; First, prepare a drying tube (with a ground glass joint) that contains anhydrous CaCl, and is fitted with a rubber stopper that will fit into a 100 ml round bottom flask. In a 100 mL round bottom flask containing a stir-bar, add tert-butylammonium chloride, ['BuNH ]CI, (1.3 g) and THF (15 ml), and then immediately stopper the flask using the stopper/drying tube fitting. Next, add sodium borohydride, NaBH. (0.2 g, weighed accurately) to the stirred suspension followed by an additional volume of THF (10 mL); be sure to stopper the flask upon complete addition. Allow the mixture to stir for 2 hours. After this time, filter (Buchner; use glass filter paper G6 Fisherbrand) the mixture (make sure the filter flask is clean; it is the filtrate that is to be collected and kept!). The product, "BuH NBHs, is very soluble in most organic solvents and thus is difficult to extract from the THF solution. The simplest method of isolating the product from the THF solution is to allow the solvent to slowly evaporate in the fumehood over 24 hrs; the product crystallizes out as small needles. However, if time permits, the THF may be removed using a water aspirator, consult with a Teaching Assistant for instructions on how to do this. Record the yield. Submit a sample (about 50 mg is sufficient) to the Lakehead University Instrumentation Laboratory (LUIL) and obtain a carbon, hydrogen and nitrogen combustion analysis. Final Report The report should include all measurements, observations, calculations and combustion data. Be sure to provide the theoretical C. H and N content calculations in your report. Using the combustion analysis, confirm the identity of your product. Include discussions on the following: (1) Explain how BH, might be prepared in situ in this reaction (hint: think of ['BuH3N]* as a BrnstedLowry acid and (BHS as a Brnsted-Lowry base and account for the production of H2). (2) Aluminum can form (AIF.T. and it can also form (AlF.J The corresponding boron analogue of the latter complex ion cannot be prepared. Why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


