Question: please answer last two question on post lab section. thanks Acid-Base Titrations 0 VS PRE-LAB QUESTIONS 1. Use the internet or your textbook as a

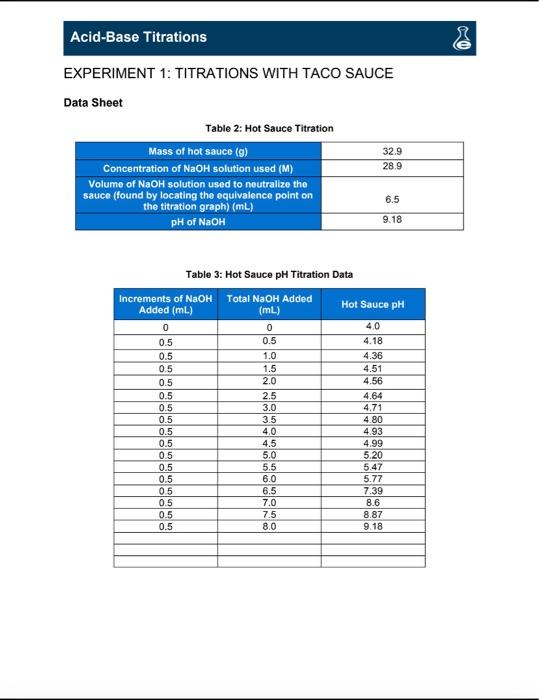
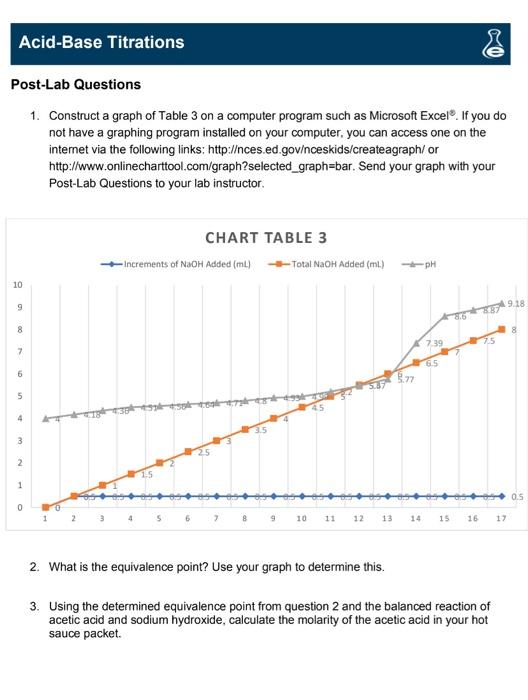
Acid-Base Titrations 0 VS PRE-LAB QUESTIONS 1. Use the internet or your textbook as a reference to compare and contrast the Arthenius Theory of acids and bases vs. the Brnsted-Lowery Theory Arrhenius Theory: Brnsted-Lowery Theory Acids contains Hion Acid-H* clonar Base-contains OH-ion Base- H+ acceptor HCI+H+C" HaH+CI NaOH. Nat +OH- NH, + H+ - NH+4 2. Use the internet or your textbook as a reference to name the following and indicate if they are an acid or a base: a. HCl-acid (H) Hydrochloric Acid D. KOH-Base (OH) Potassium Hydroxide C. HNO, Acid (H+) Nitric Acid d. Mg(OH). Base (-OH) Magnesium Hydroxide Acid-Base Titrations 0 EXPERIMENT 1: TITRATIONS WITH TACO SAUCE Data Sheet Table 2: Hot Sauce Titration Mass of hot sauce (9) Concentration of NaOH solution used (M) Volume of NaOH solution used to neutralize the sauce (found by locating the oquivalence point on the titration graph) (ml) pH of NaOH 32.9 28.9 6.5 9.18 (mL) Table 3: Hot Sauce pH Titration Data Increments of NaOH Total NaOH Added Added (mL) Hot Sauce pH 0 0 4.0 0.5 0.5 4.18 0.5 1.0 4.36 0.5 1.5 4.51 2.0 4.56 0.5 2.5 4.64 0.5 3.0 4.71 0.5 3.5 4.80 0.5 4.0 4.93 0.5 4.5 4.99 0.5 5.0 5.20 0.5 5.5 5.47 0.5 6.0 5.77 6.5 7.39 0.5 7.0 8.6 0.5 7.5 8.87 0.5 8,0 9 18 Acid-Base Titrations Post-Lab Questions 1. Construct a graph of Table 3 on a computer program such as Microsoft Excel. If you do not have a graphing program installed on your computer, you can access one on the internet via the following links: http:/ces.ed.govinceskids/createagraph/or http://www.onlinecharttool.com/graph?selected_graph=bar. Send your graph with your Post-Lab Questions to your lab instructor CHART TABLE 3 -Increments of NaOH Added (ml) - Total NaOH Added (m.) pH 10 9 9.18 86 987 8 8 739 25 7 GS 5 4 3 2 1 0.5 0 1 15 16 17 2. What is the equivalence point? Use your graph to determine this. 3. Using the determined equivalence point from question 2 and the balanced reaction of acetic acid and sodium hydroxide, calculate the molarity of the acetic acid in your hot sauce packet
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


