Question: PLEASE answer only part 5 of this Question. AND PLEASE also look at the Four Graphs.They are oart of 5. I am attaching the Question
PLEASE answer only part 5 of this Question. AND PLEASE also look at the Four Graphs.They are oart of 5. I am attaching the Question and the Four Graphs.

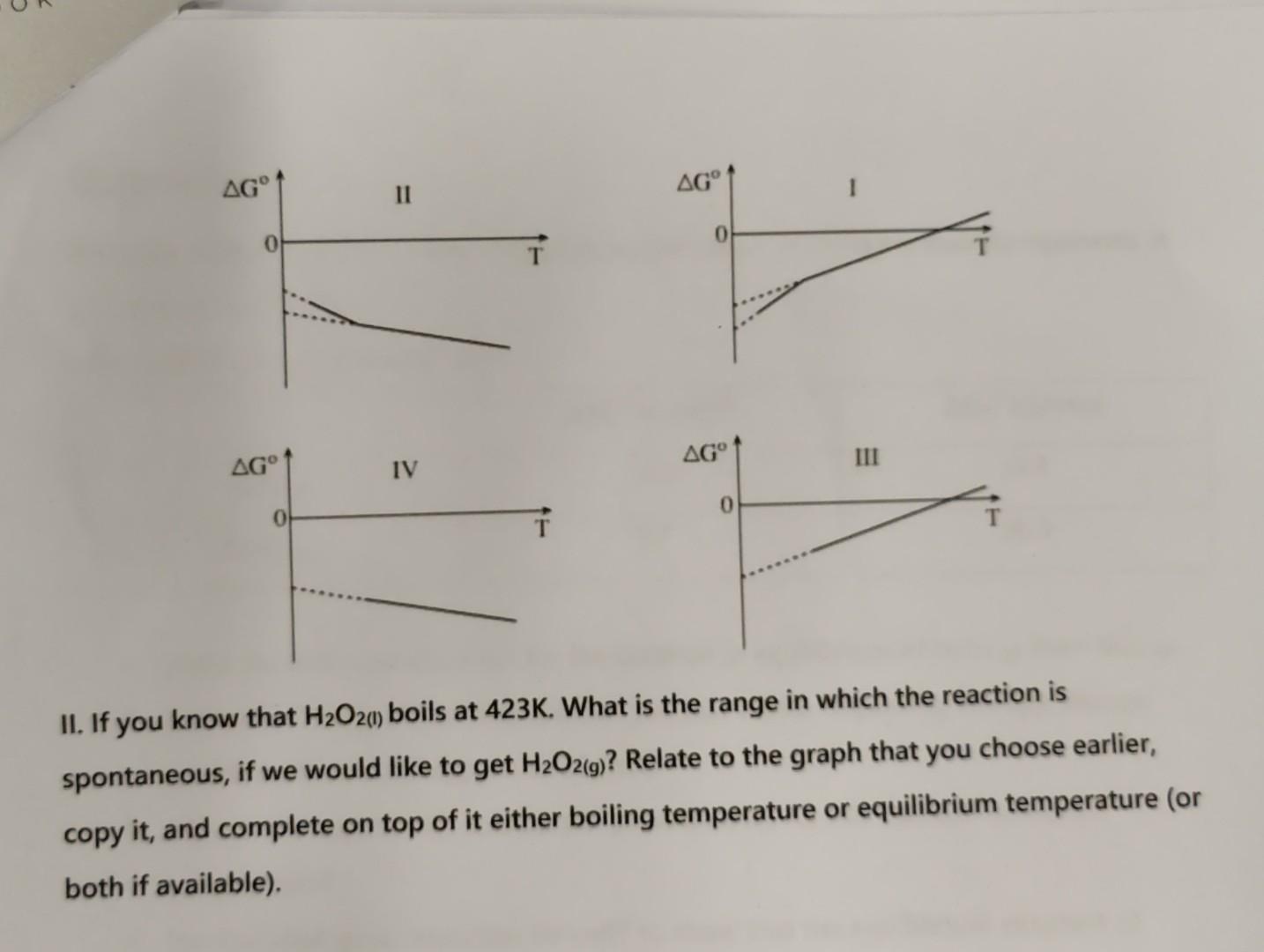
PLEASE don't forget the question under the graphs.
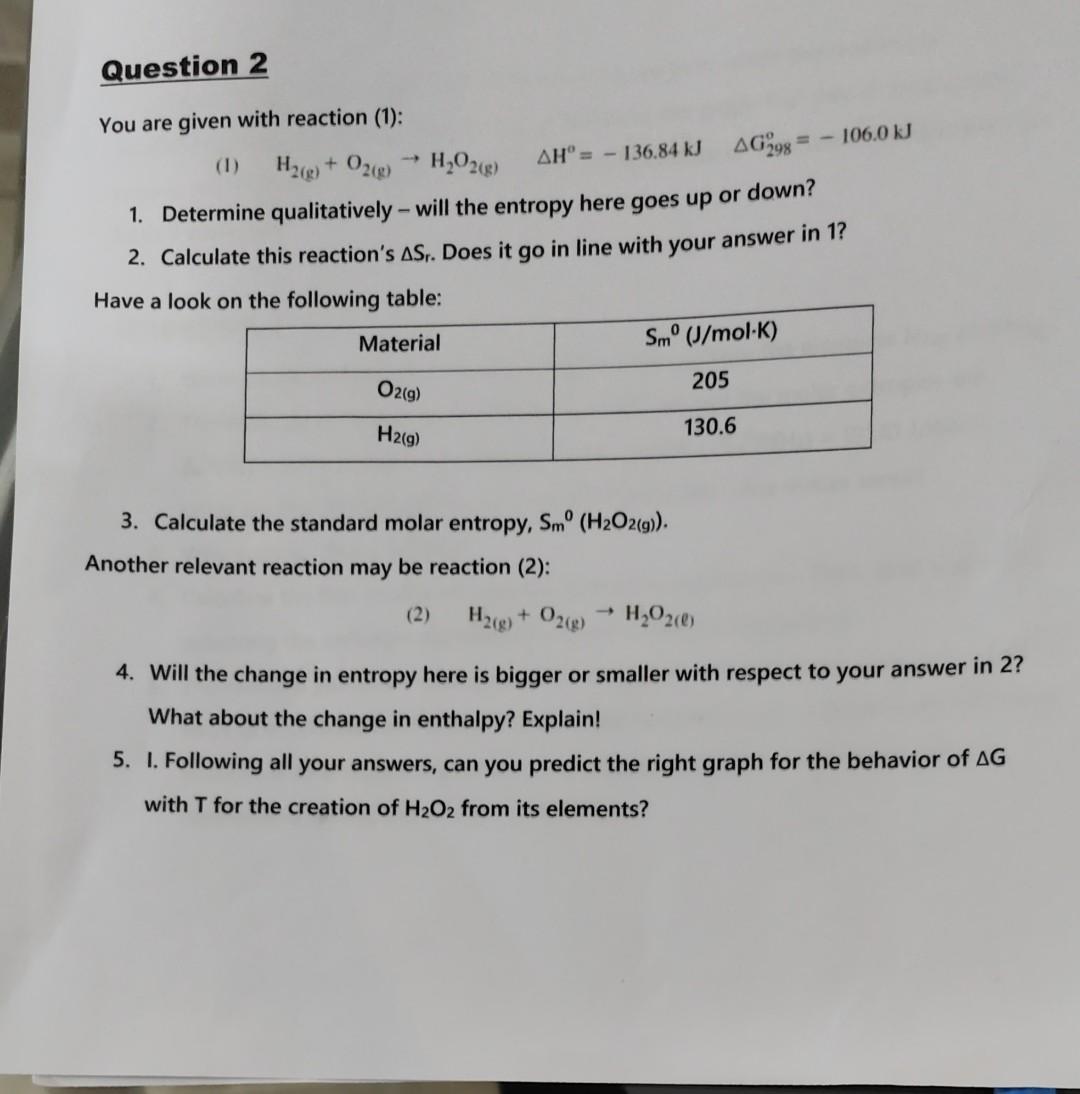
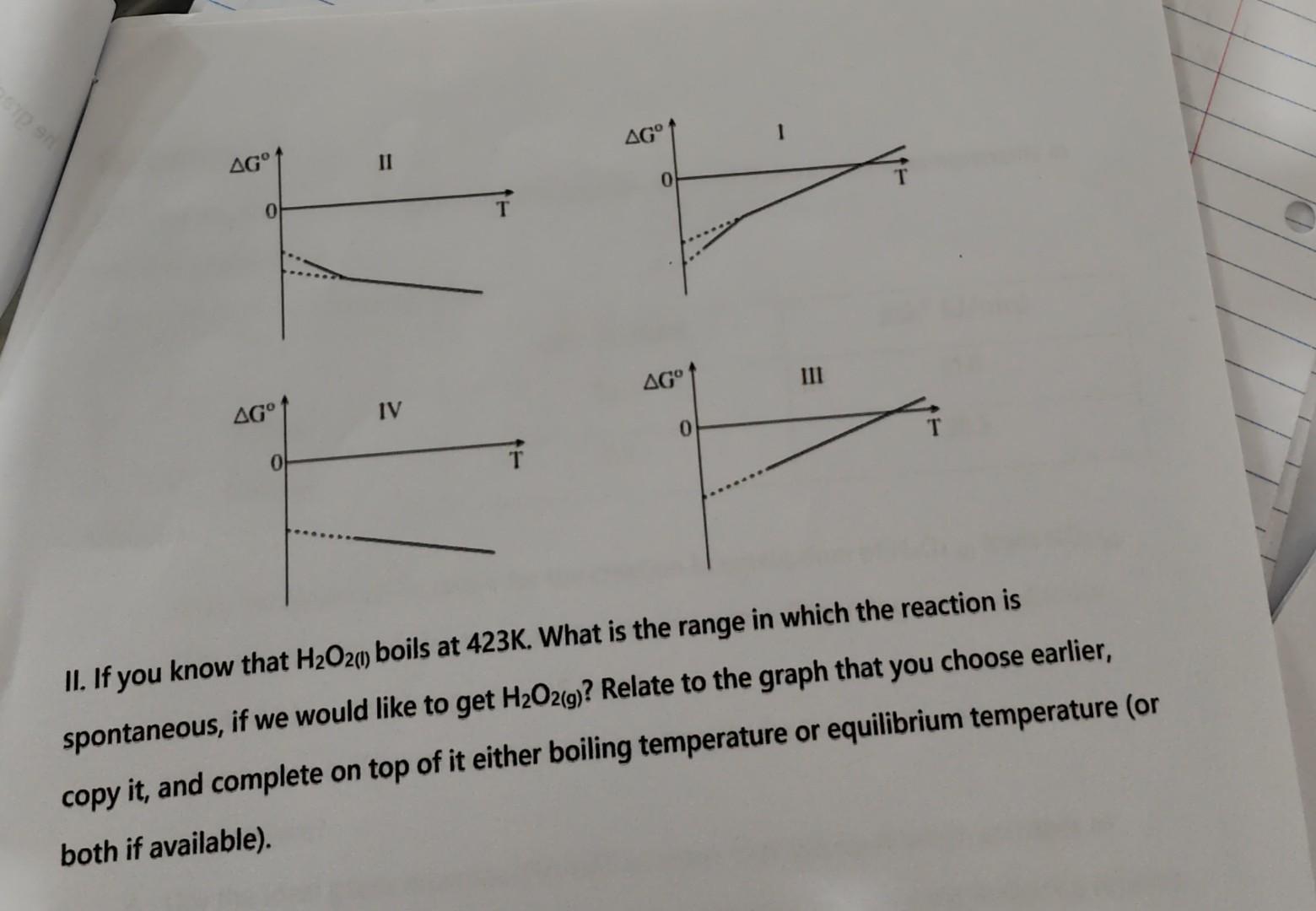
I need Help for 5 I and 5 II
You are given with reaction (1): (1) H2(g)+O2(g)H2O2(g)H=136.84kJG298o=106.0kJ 1. Determine qualitatively - will the entropy here goes up or down? 2. Calculate this reaction's Sr. Does it go in line with your answer in 1 ? Have a look on the following table: 3. Calculate the standard molar entropy, Sm0(H2O2(g)). Another relevant reaction may be reaction (2): (2) H2(g)+O2(g)H2O2() 4. Will the change in entropy here is bigger or smaller with respect to your answer in 2 ? What about the change in enthalpy? Explain! 5. I. Following all your answers, can you predict the right graph for the behavior of G with T for the creation of H2O2 from its elements? II. If you know that H2O2(1) boils at 423K. What is the range in which the reaction is spontaneous, if we would like to get H2O2(g) ? Relate to the graph that you choose earlier, copy it, and complete on top of it either boiling temperature or equilibrium temperature (or both if available). You are given with reaction (1): (1) H2(g)+O2(g)H2O2(g)H=136.84kJG298o=106.0kJ 1. Determine qualitatively - will the entropy here goes up or down? 2. Calculate this reaction's Sr. Does it go in line with your answer in 1 ? Have a look on the following table: 3. Calculate the standard molar entropy, Sm0(H2O2(g)). Another relevant reaction may be reaction (2): (2) H2(g)+O2(g)H2O2() 4. Will the change in entropy here is bigger or smaller with respect to your answer in 2 ? What about the change in enthalpy? Explain! 5. I. Following all your answers, can you predict the right graph for the behavior of G with T for the creation of H2O2 from its elements? H2O2(g) ? Relate to the graph that you choose earlier, copy it, and complete on top of it either boiling temperature or equilibrium temperature (or both if available)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


