Question: Please answer part A and B. A solution of 5.0g benzoic acid (C6HCOOH) in 100.0g of carbon tetrachloride (Kb=5.03 C/m;BP=76.5C ) has a boiling point
Please answer part A and B.
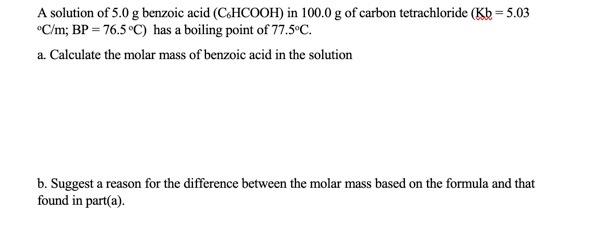
A solution of 5.0g benzoic acid (C6HCOOH) in 100.0g of carbon tetrachloride (Kb=5.03 C/m;BP=76.5C ) has a boiling point of 77.5C. a. Calculate the molar mass of benzoic acid in the solution b. Suggest a reason for the difference between the molar mass based on the formula and that found in part(a)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


