Question: Please answer part a and part b Consider the Severinghaus electrode shown below for measurement of pCO2. The solution internal to the glass membrane is
Please answer part a and part b
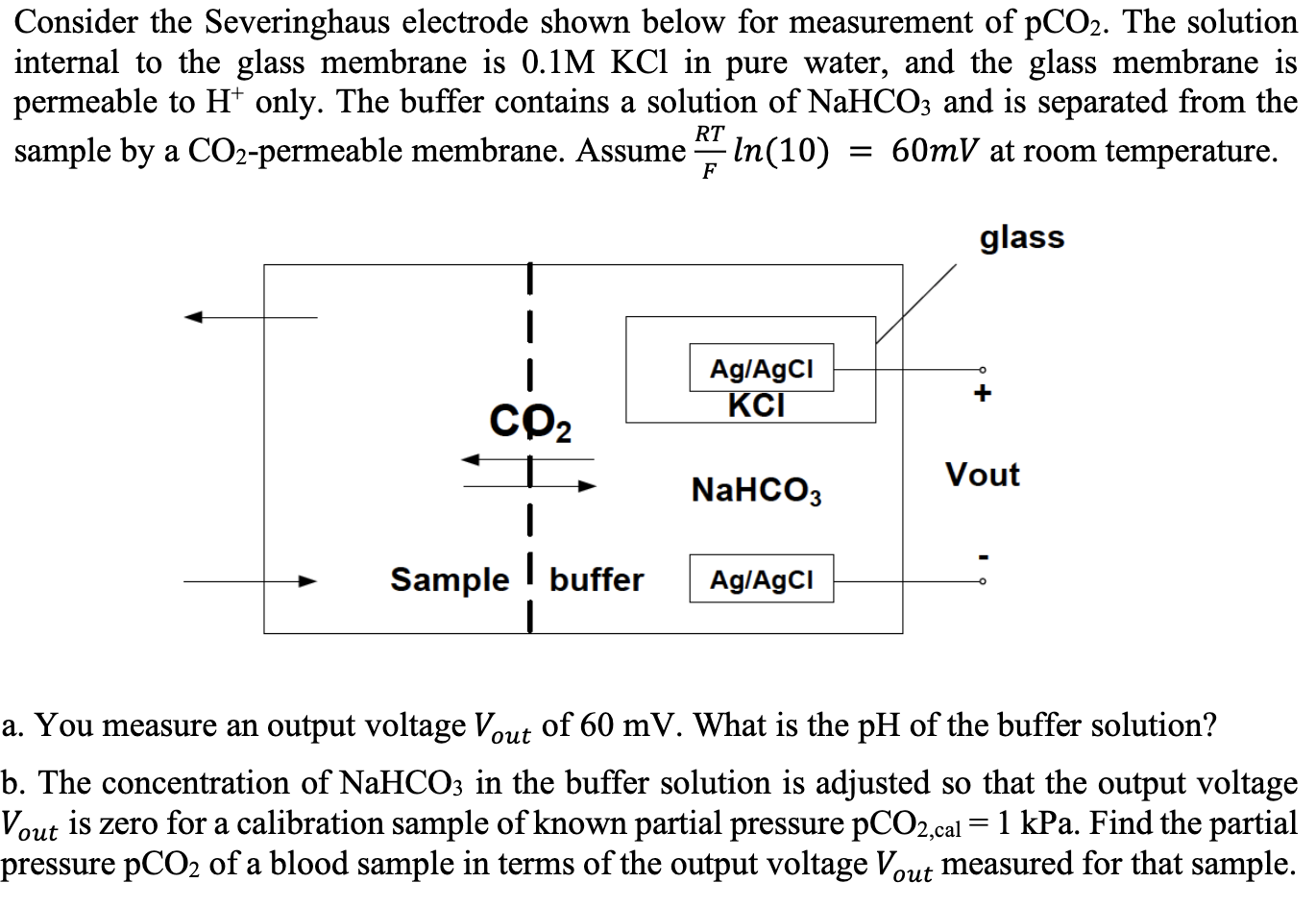
Consider the Severinghaus electrode shown below for measurement of pCO2. The solution internal to the glass membrane is 0.1MKCl in pure water, and the glass membrane is permeable to H+only. The buffer contains a solution of NaHCO3 and is separated from the sample by a CO2-permeable membrane. Assume FRTln(10)=60mV at room temperature. a. You measure an output voltage Vout of 60mV. What is the pH of the buffer solution? b. The concentration of NaHCO3 in the buffer solution is adjusted so that the output voltage Vout is zero for a calibration sample of known partial pressure pCO2,cal=1kPa. Find the partial pressure pCO2 of a blood sample in terms of the output voltage Vout measured for that sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


