Question: Please answer part B and C using the information above in Part A and the additional information given, thanks! Gibbs Energy: Temperature Dependence The chemical
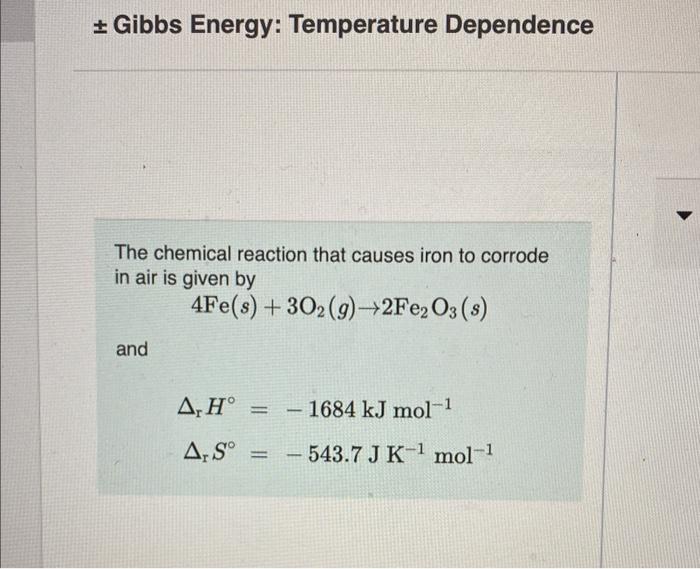
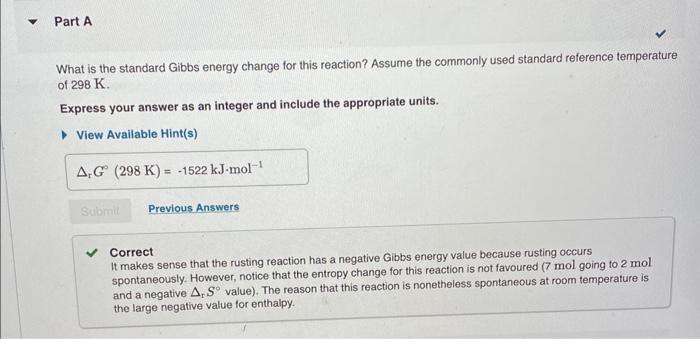
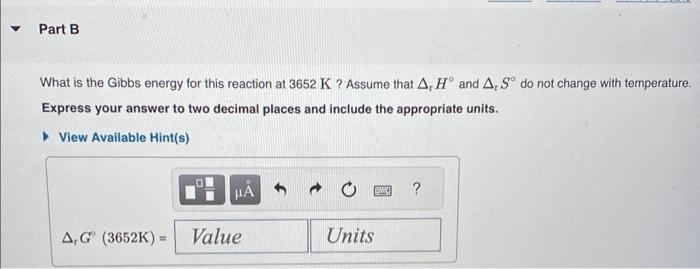

Gibbs Energy: Temperature Dependence The chemical reaction that causes iron to corrode in air is given by 4Fe(s)+3O2(g)2Fe2O3(s) and rH=1684kJmol1rS=543.7JK1mol1 What is the standard Gibbs energy change for this reaction? Assume the commonly used standard reference temperature of 298K. Express your answer as an integer and include the appropriate units. Correct It makes sense that the rusting reaction has a negative Gibbs energy value because rusting occurs spontaneously. However, notice that the entropy change for this reaction is not favoured (7mol going to 2mol and a negative rS value). The reason that this reaction is nonetheless spontaneous at room temperature is the large negative value for enthalpy. What is the Gibbs energy for this reaction at 3652K ? Assume that rH and rS do not change with temperature. Express your answer to two decimal places and include the appropriate units. The standard Gibbs energy change, rG, applies only when the reactants and products are in their standard states. Assume that the above corrosion reaction is carried out in a flask where the reactants and products are in their standard states and the values of rH and rS do not change with temperature. At what temperature, Teq, do the forward and reverse corrosion reactions occur in equilibrium? Express your answer as an integer and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


