Question: please answer part B precipitato will form At 35 the solution is supersaturated, but by 0C the solution is unsa precipitato will form MISSED THIS?
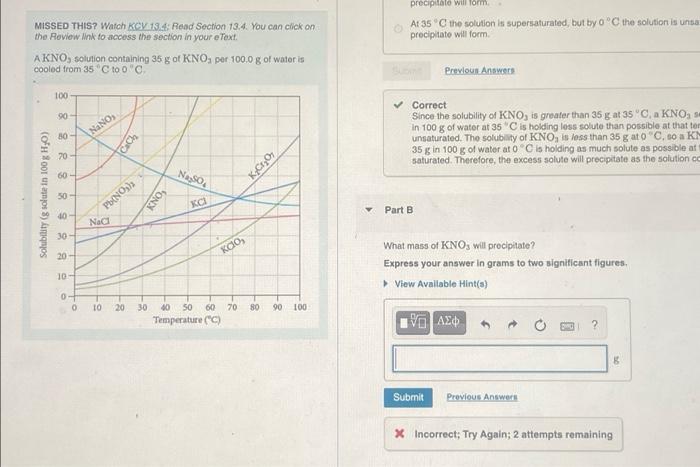
precipitato will form At 35 the solution is supersaturated, but by 0C the solution is unsa precipitato will form MISSED THIS? Watch KCV 13.4. Road Section 13.4. You can click on the Review link to access the section in your e Text AKNO, solution containing 35 g of KNO, per 100,0 g of water is cooled from 35 C too Previous Answers 100 90 80- NaNO, Cach Correct Since the solubility of KNO, is greater than 35 g at 35 C, a KNO, s in 100 g of water at 35 C is holding loss solute than possible at that to unsaturated. The solubility of KNO, is less than 35 gato", so a K- 35 g in 100 g of water ato is holding as much solute as possible at saturated. Therefore, the excess solute will precipitate as the solution of 20 60 - Naso KCrO (of door tass) angos 50 Pb(NO3)2 KNO, Part B 40 - 30 20- Kao, What mass of KNO3 will procipitate? Express your answer in grams to two significant figures View Available Hint() 10 0- 0 - 10 70 80 90 100 20 30 60 50 60 Temperature ( 10 AED ? Submit Previous Answers * Incorrect; Try Again; 2 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


