Question: Please answer parts A through E below, including the diagram for part B . Thank you! ( 5 0 points ) Let us calculate the
Please answer parts A through E below, including the diagram for part B Thank you!
points Let us calculate the compositions and quantities of the gas and liquid when mole of the following mixture is brought to equilibrium at and psia. Raoult's law is assumed to be valid in this question.
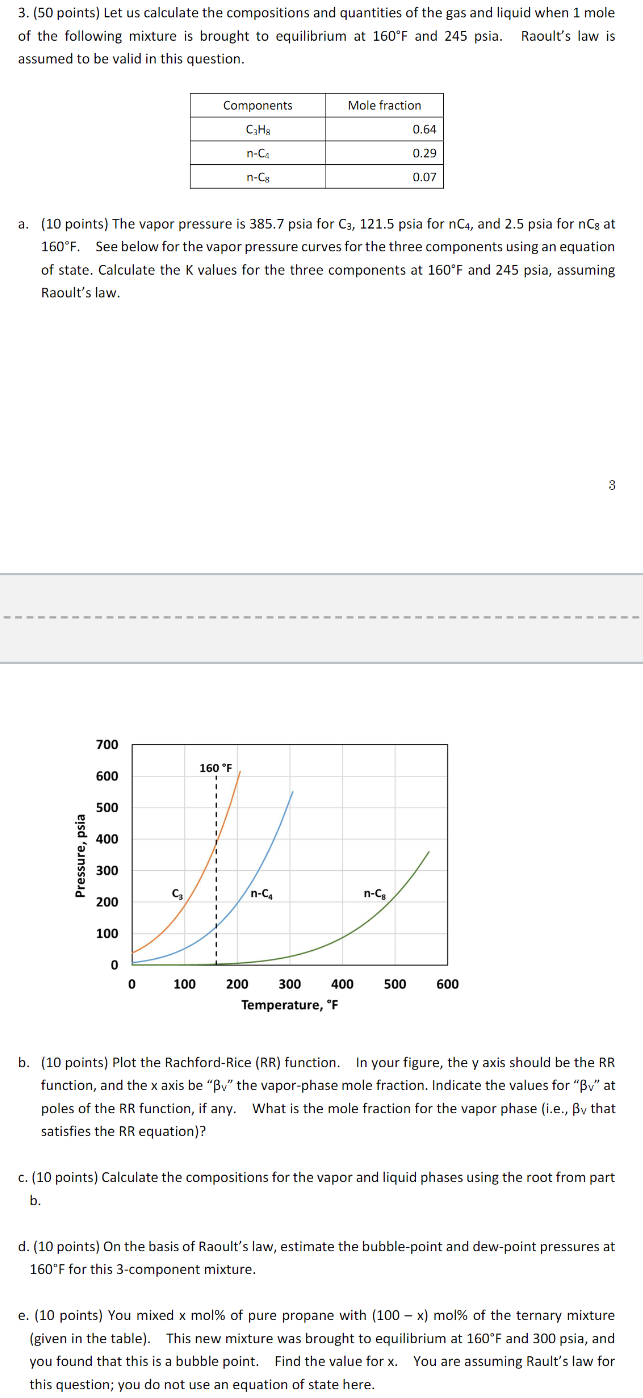
a points The vapor pressure is psia for for and psia for at See below for the vapor pressure curves for the three components using an equation of state. Calculate the values for the three components at and psia, assuming Raoult's law.
b points Plot the RachfordRice RR function. In your figure, the y axis should be the RR function, and the axis be the vaporphase mole fraction. Indicate the values for at poles of the RR function, if any. What is the mole fraction for the vapor phase ie that satisfies the RR equation
c points Calculate the compositions for the vapor and liquid phases using the root from part b
d points On the basis of Raoult's law, estimate the bubblepoint and dewpoint pressures at for this component mixture.
e points You mixed mol of pure propane with mol of the ternary mixture given in the table This new mixture was brought to equilibrium at and and you found that this is a bubble point. Find the value for You are assuming Rault's law for this question; you do not use an equation of state here.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


