Question: please answer post lab questions and explain how you got the answer so i can use it for future references, thank you! EXPERIMENT 1: EQUILIBRIUM
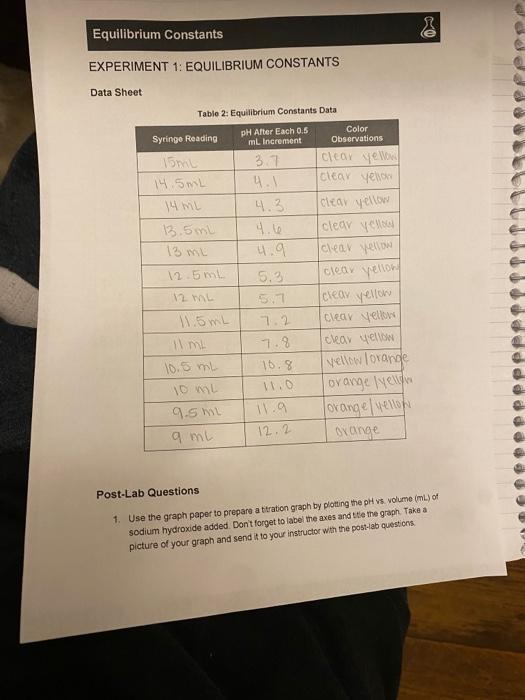

EXPERIMENT 1: EQUILIBRIUM CONSTANTS Data Sheet Tahin 2- Faulilbrium Constants Data Post-Lab Questions 1. Use the graph paper to prepare a tiration graph by ploting the pH vs volume (mL) of sodium hydroxide added. Dont forget to label the axes and ttie the graph. Take a picture of your graph and send it to your instructor whth the post-lab questicns. 2. From your graph, determine the volume of sodium hydroxide needed to reach the equivalence point in the titration. 3. Calculate the volume needed to reach the half-equivalence point in the titration. 4. Find this half-equivalence point on the graph and determine its corresponding pH. 5. Use the half-equivalence point pH value to find the experimental pKa. To solve, recall that: pH=pKa+log[acid][base] 6. Determine the Ka of acetic acid. Recall that: Ka=[HHA][H2O+][A]pK=log(Ka) 7. Look up the actual value for the equilibrium constant (Ka; acid ionization constant) for acetic acid (use a website like the Lab Rat as a reliable resource). Compare your answer to the value in the literature. Do you notice any vaniation? If so, why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


