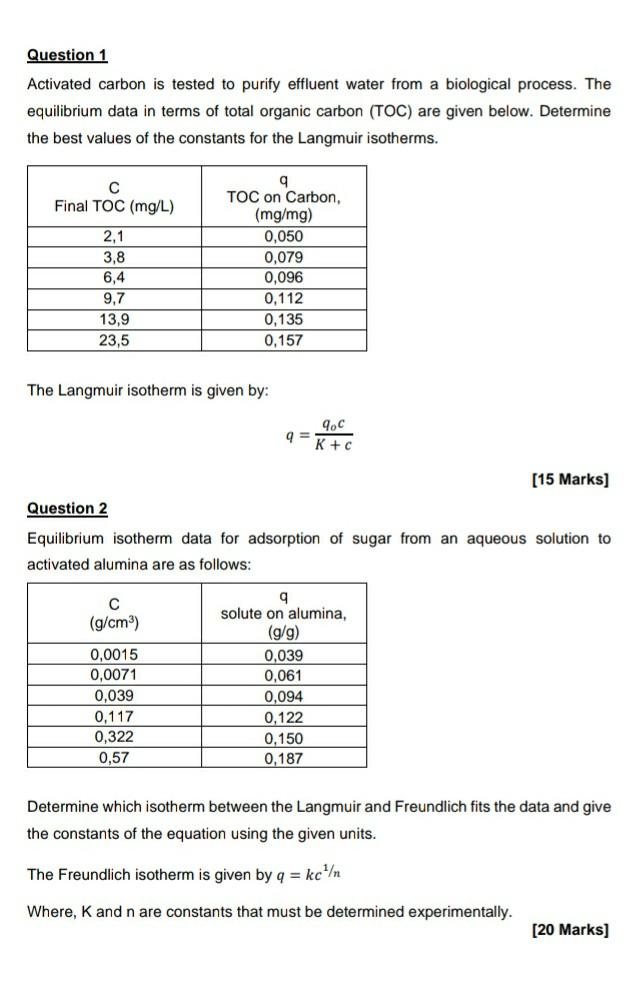
Question: please answer Question 1 Activated carbon is tested to purify effluent water from a biological process. The equilibrium data in terms of total organic carbon
please answer
Question 1 Activated carbon is tested to purify effluent water from a biological process. The equilibrium data in terms of total organic carbon (TOC) are given below. Determine the best values of the constants for the Langmuir isotherms. Final TOC (mg/L) 2,1 3,8 6,4 9,7 13.9 23,5 q TOC on Carbon, (mg/mg) 0,050 0,079 0,096 0,112 0,135 0,157 The Langmuir isotherm is given by: 9.C = K + c [15 Marks] Question 2 Equilibrium isotherm data for adsorption of sugar from an aqueous solution to activated alumina are as follows: (g/cm) 0,0015 0.0071 0,039 0,117 0.322 0,57 9 solute on alumina, (g/g) 0,039 0,061 0,094 0,122 0.150 0,187 Determine which isotherm between the Langmuir and Freundlich fits the data and give the constants of the equation using the given units. The Freundlich isotherm is given by q = kc Where, K and n are constants that must be determined experimentally. [20 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


