Question: please answer question 13.48 but use methanol (1) / water (2) system and apply NRTL equation data table. 13.47. For one of the binary systems
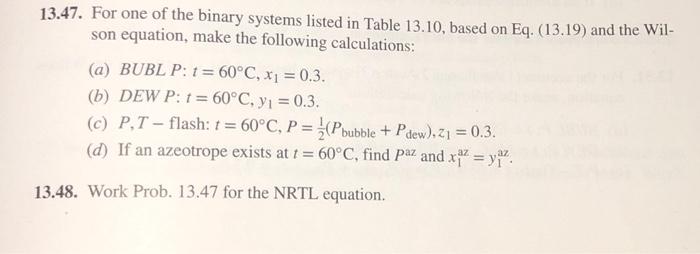
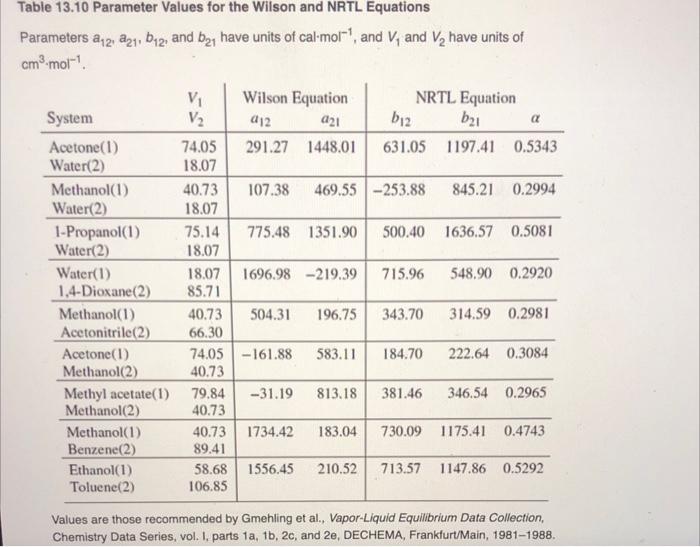
13.47. For one of the binary systems listed in Table 13.10, based on Eq. (13.19) and the Wil- son equation, make the following calculations: (a) BUBL P: 1= 60C, x = 0.3. (b) DEW P: 1 = 60C, y = 0.3. (c) P,T-flash: t = 60C, P = (Pbubble + Pdew),21 = 0.3. (d) If an azeotrope exists at t = 60C, find Paz and xaz = y. 13.48. Work Prob. 13.47 for the NRTL equation. Table 13.10 Parameter Values for the Wilson and NRTL Equations Parameters a12, 21, b12, and b21 have units of cal-mol-, and V, and V have units of cm-mol- V Wilson Equation NRTL Equation b1 System V a12 a21 b12 a 74.05 291.27 1448.01 631.05 1197.41 0.5343 Acetone(1) Water(2) 18.07 Methanol(1) 40.73 107.38 469.55 -253.88 845.21 0.2994 Water(2) 18.07 1-Propanol(1) 75.14 775.48 1351.90 500.40 1636.57 0.5081 Water(2) 18.07 Water(1) 18.07 1696.98 -219.39 715.96 548.90 0.2920 1,4-Dioxane(2) 85.71 Methanol(1) 314.59 0.2981 40.73 504.31 196.75 343.70 66.30 Acetonitrile (2) Acetone(1) 74.05-161.88 583.11 184.70 222.64 0.3084 Methanol(2) 40.73 Methyl acetate (1) 79.84 -31.19 813.18 381.46 346.54 0.2965 Methanol(2) 40.73 Methanol(1) 40.73 1734.42 183.04 730.09 1175.41 0.4743 Benzene(2) 89.41 Ethanol(1) 58.68 1556.45 210.52 713.57 1147.86 0.5292 Toluene(2) 106.85 Values are those recommended by Gmehling et al., Vapor-Liquid Equilibrium Data Collection, Chemistry Data Series, vol. I, parts 1a, 1b, 2c, and 2e, DECHEMA, Frankfurt/Main, 1981-1988
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


