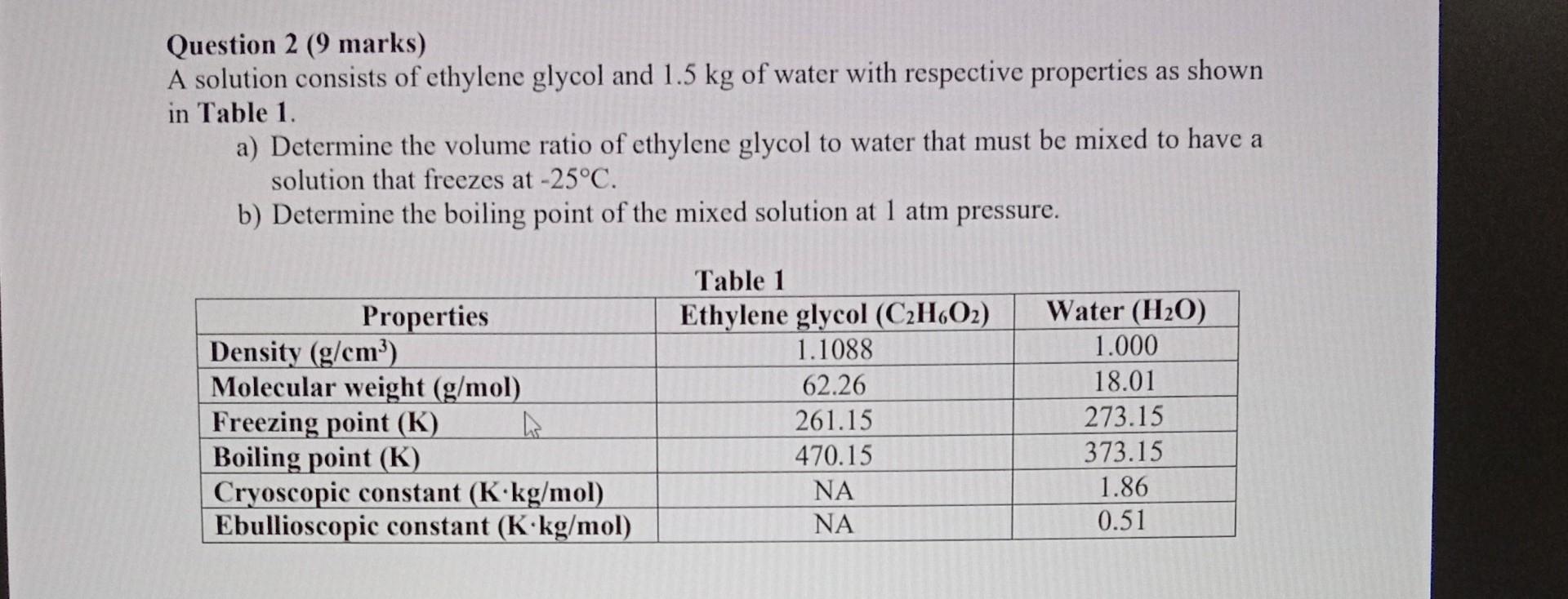
Question: please answer Question 2 (9 marks) A solution consists of ethylene glycol and 1.5kg of water with respective properties as shown in Table 1. a)
please answer
Question 2 (9 marks) A solution consists of ethylene glycol and 1.5kg of water with respective properties as shown in Table 1. a) Determine the volume ratio of ethylene glycol to water that must be mixed to have a solution that freezes at 25C. b) Determine the boiling point of the mixed solution at 1atm pressure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


