Question: please answer question 2 urgently 1.0 0.9 0.8 0.7 0.6 X 0.5 T=45 C -T=35 -O-T=25 0.4 0.2 0.1- 40 80 100 120 . 0.00

please answer question 2 urgently
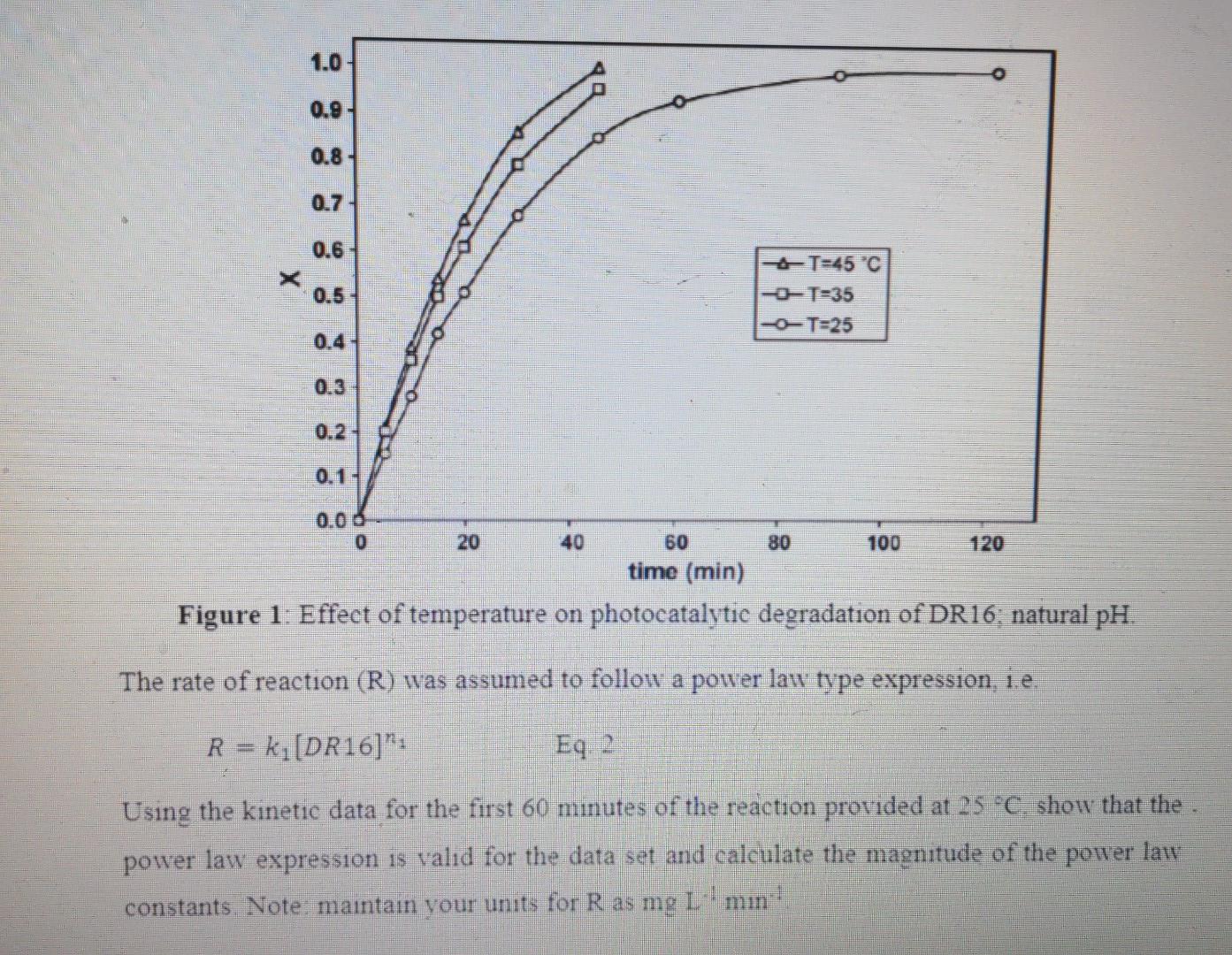
1.0 0.9 0.8 0.7 0.6 X 0.5 T=45 C -T=35 -O-T=25 0.4 0.2 0.1- 40 80 100 120 . 0.00 0 20 60 time (min) Figure 1: Effect of temperature on photocatalytic degradation of DR16: natural pH. The rate of reaction (R) was assumed to follow a power law type expression, i.e. R = ki[DR16]": Eq. 2 Using the kinetic data for the first 60 minutes of the reaction provided at 25 C. show that the power law expression is valid for the data set and calculate the magnitude of the power late constants. Note: maintain your units for R as mg L-' min The kinetic rate for the decomposition of direct red 16 (DR16) effluent from a dye house has been studied in a batch reactor. The degradation efficiency (X) for the reaction was described as Eq. 1: [DR16).-(DR16] X = [DR16). Eq. 1 where [DR16]o is the initial concentration of the dye (typically around 30 mg L-) and [DR16) is the concentration of the dve at a future time. The effect of temperature at natural pH on the degradation efficiency is provided on figure 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


