Question: please answer question 6. its a multi step question that covers many sections of different chapters. Im having trouble believing that the answers I arrived
please answer question 6. its a multi step question that covers many sections of different chapters. Im having trouble believing that the answers I arrived too are incorrect. Please solve each part throughly with explanations. Also, please do not submit incorrect work that will not help me fix any errors that I may have made. Look forward to growing. Thanks in advance! :)
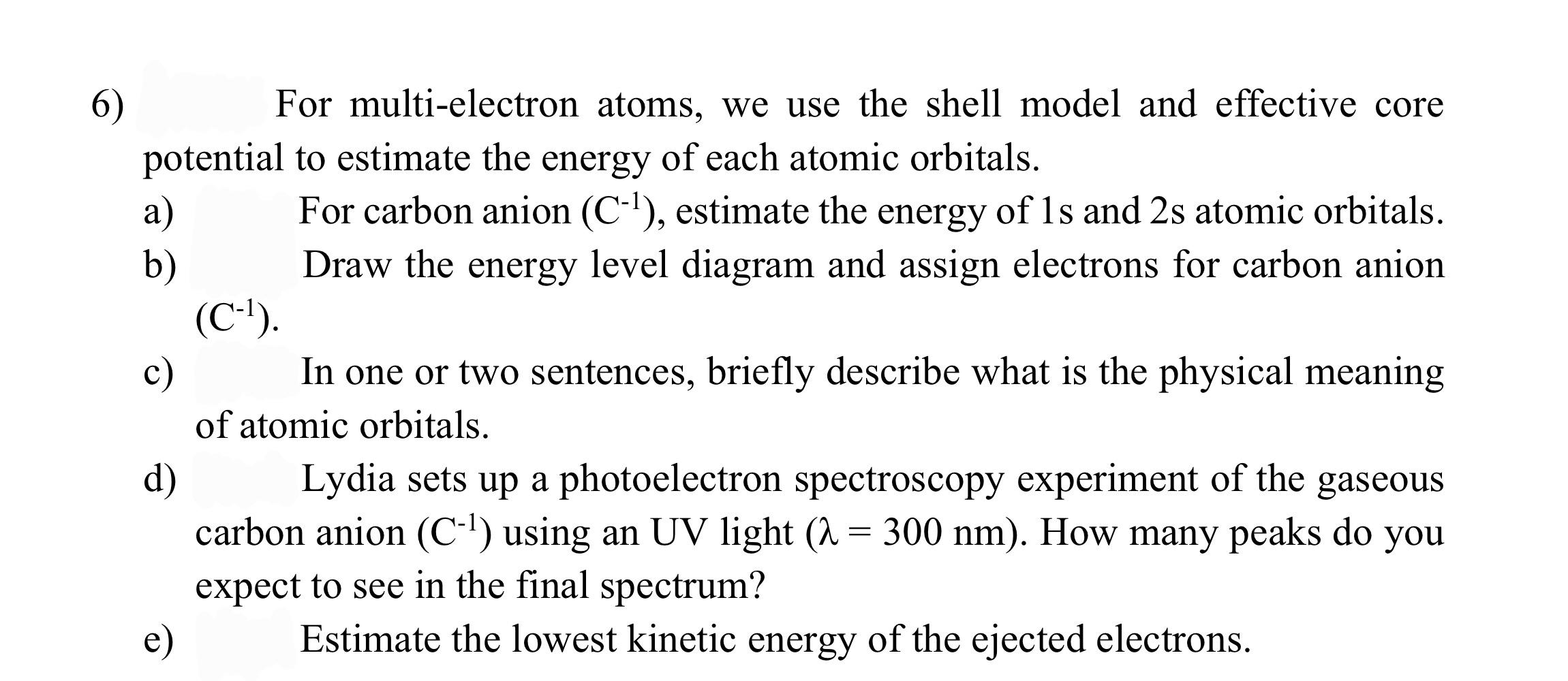
6) For multi-electron atoms, we use the shell model and effective core potential to estimate the energy of each atomic orbitals. a) For carbon anion (C-?), estimate the energy of ls and 2s atomic orbitals. b) Draw the energy level diagram and assign electrons for carbon anion (C-1). c) In one or two sentences, briefly describe what is the physical meaning of atomic orbitals. d) Lydia sets up a photoelectron spectroscopy experiment of the gaseous carbon anion (C-1) using an UV light (a = 300 nm). How many peaks do you expect to see in the final spectrum? e) Estimate the lowest kinetic energy of the ejected electrons. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


