Question: please answer questions b,c,d (b) What is the theoretieal yield of CH3OH in grams? 2pts (c) How much of the reagent present in excess is

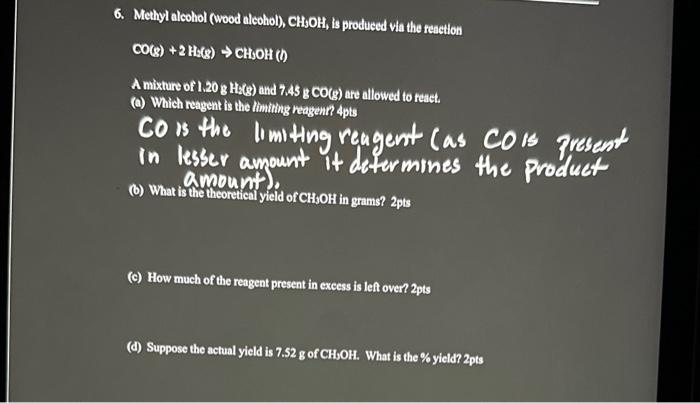
(b) What is the theoretieal yield of CH3OH in grams? 2pts (c) How much of the reagent present in excess is left over? 2 pts (d) Suppose the actual yield is 7.52g of CH,OH. What is the \% yield? 2pts 6. Methyl alcohol (wood alcohol), CH;OH, is produced via the reaction CO(g)+2HS(g)CHSOH(l) A mixture of 1.20gHs(g) and 7.45gCO(g) are allowed to reach. (a) Which reagent is the limiting reagent? 4pts CO is the lomiting reagent Cas COrs Fresent in lesser amount it determines the product amoonini), (b) What is the theoretical yield of CHJOH in grams? 2pts (c) How much of the reagent present in excess is left oven 2pts (d) Suppose the actual yield is 7.52g of CH,OH. What is the \% yicld 2pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


