Question: please answer questions iii. through v. using the same form as used in questions i. and ii. I attached a photo of the diagram as

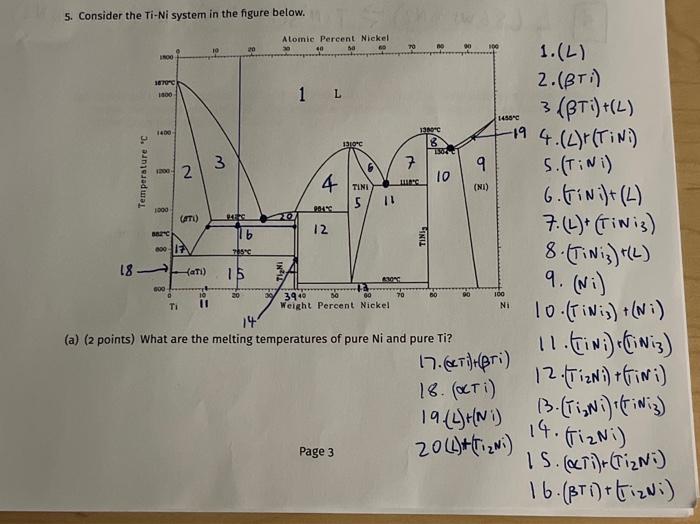
(f) The following questions deal with an alloy of 20wt%Ni,80wt% Ti that is slowly cooled from the completely liquid state. i. (2 points) At what temperature does the first solid form and what is its composition? ii. (2 points) At what temperature does the last liquid exist and what is its composition? 942a0CL(28w+%Ni) iii. (8 points) At a temperature of 900C what phases are present in the alloy, what are their compositions and what is the relative amount of each phase? 2,16,14,12,5,11,10,9 iv. ( 4 points) At a temperature of 900C what is the relative amount of primary in your alloy? v. ( 2 points) At a temperature of 900C what is the relative amount of in the eutectic? 5. Consider the Ti-Ni system in the figure below. (a) (2 points) What are the melting temperatures of pure Ni and pure Ti
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


