Question: Please Answer Quick!!! ASAP!!! 3. A 30.00 mL sample of an unknown H3POs solution is titrated with a 0.100 M KOH solution. The equivalence point
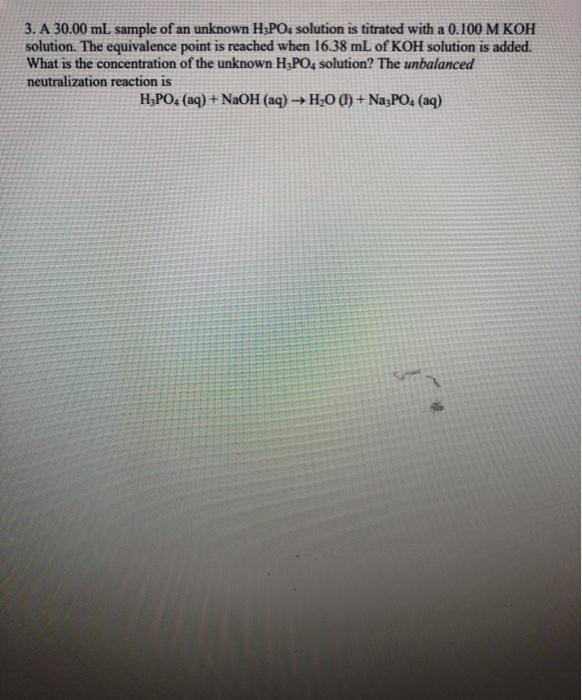
3. A 30.00 mL sample of an unknown H3POs solution is titrated with a 0.100 M KOH solution. The equivalence point is reached when 16.38 mL of KOH solution is added. What is the concentration of the unknown H3PO4 solution? The unbalanced neutralization reaction is H3PO4 (aq) + NaOH(aq) H2O(l) + Na3PO4 (aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


