Question: PLEASE ANSWER QUICKLY! The graph shows differenr methods of formulas rhat need to be solved and the information provided is for those questions Ke is
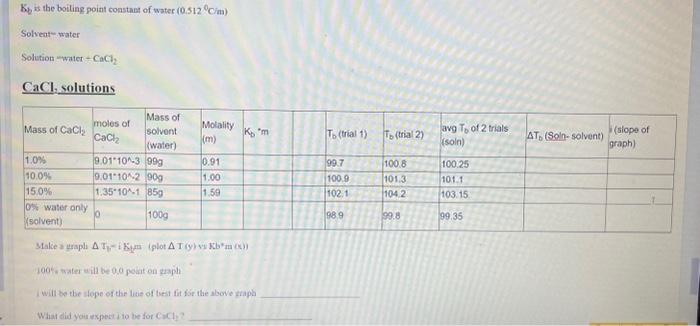
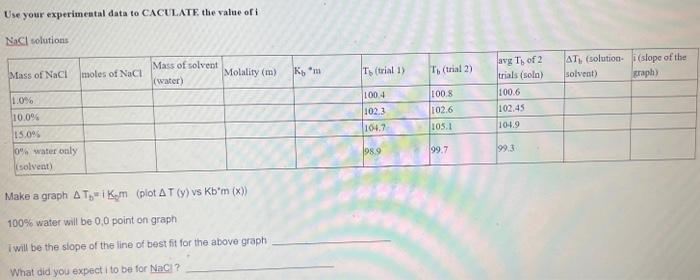
Ke is the boiling point comstant of water (0.512C/m) Solveat - water Solvtion water CaCl2 CaCl,Co)Bliinn Make a raphi T1 i Krma (plot I(y) vy Jb*ia (c)) 100% s tsater eill be 0,0 polat on gaspl I wilf be the dege of the lieie of theit tit d5e the aloove ziapl What did yourkper it to be for CaClC ? Use your experimental data to CACULATE. the value of i NaCl solutions Make a graph Tb=iKm (piot T(y) vs Kbm(x) ) 10086 water will be 0,0 point on graph I will be the siope of the line of best fit for the above graph What did you expect i to be for Nacl? Ke is the boiling point comstant of water (0.512C/m) Solveat - water Solvtion water CaCl2 CaCl,Co)Bliinn Make a raphi T1 i Krma (plot I(y) vy Jb*ia (c)) 100% s tsater eill be 0,0 polat on gaspl I wilf be the dege of the lieie of theit tit d5e the aloove ziapl What did yourkper it to be for CaClC ? Use your experimental data to CACULATE. the value of i NaCl solutions Make a graph Tb=iKm (piot T(y) vs Kbm(x) ) 10086 water will be 0,0 point on graph I will be the siope of the line of best fit for the above graph What did you expect i to be for Nacl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


