Question: Please answer the following questions below. A) Choose a base from the pKa table that will first only deprotonate the oxygen followed by another base
Please answer the following questions below.
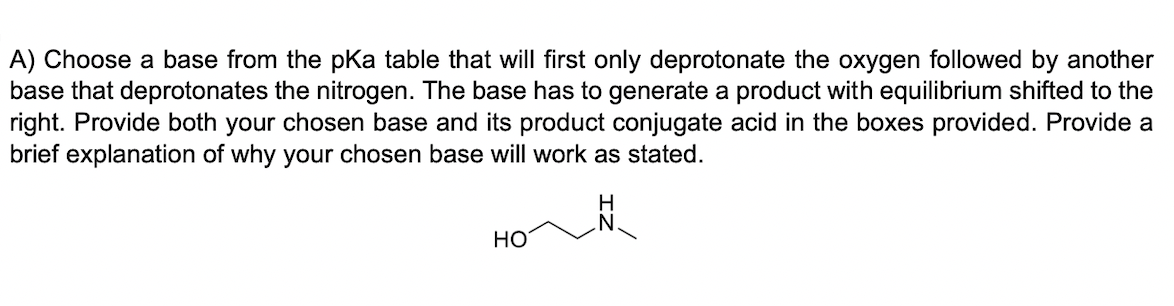

A) Choose a base from the pKa table that will first only deprotonate the oxygen followed by another base that deprotonates the nitrogen. The base has to generate a product with equilibrium shifted to the right. Provide both your chosen base and its product conjugate acid in the boxes provided. Provide a brief explanation of why your chosen base will work as stated. Two resonance structures of CH2NOCH3 (Hint: CH2 bonded to N,N bonded to O and CH3 ). Follow the more detailed Lewis structure approach. a) Calculate the total valence electrons, b) Assign center and surrounding atoms, c) make a single bond among atoms, d) calculate remaining valence es unused (each bond =2 es), e) add lone pairs to meet octet on surrounding atoms first until no more valence electrons remain, f) try to redraw structure for best octets number on atoms, g) calculate charges or potential resonance)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


