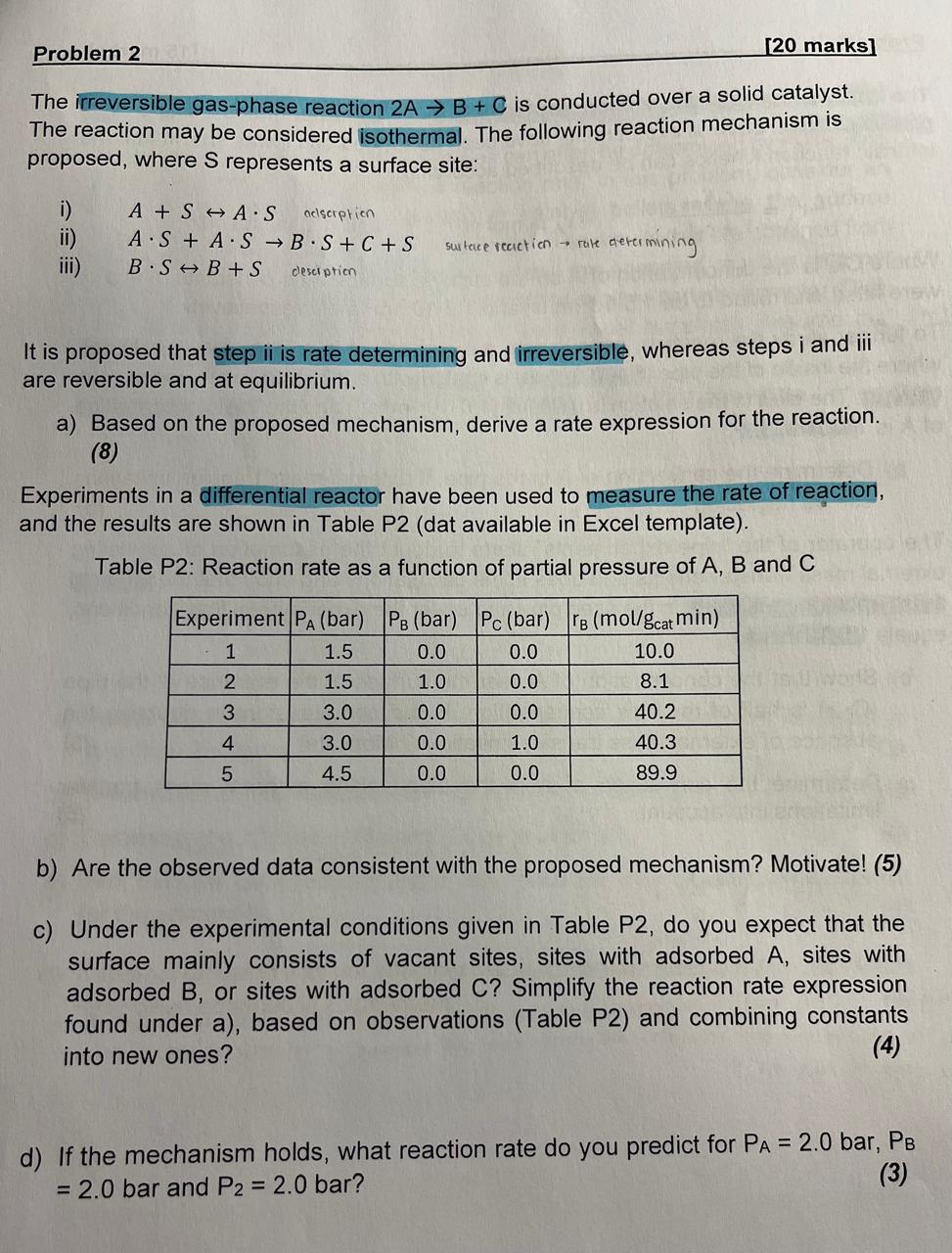
Question: Please answer the question complete and use polymath or excel in case needed. The irreversible gas - phase reaction 2 A B + C is
Please answer the question complete and use polymath or excel in case needed.
The irreversible gasphase reaction is conducted over a solid catalyst.
The reaction may be considered isothermal. The following reaction mechanism is
proposed, where represents a surface site:
iSharrA alscerption
ii
iii
surtace reaction rake aterermining
It is proposed that step ii is rate determining and irreversible, whereas steps i and iii
are reversible and at equilibrium.
a Based on the proposed mechanism, derive a rate expression for the reaction.
Experiments in a differential reactor have been used to measure the rate of reaction,
and the results are shown in Table Pdat available in Excel template
Table P: Reaction rate as a function of partial pressure of and
b Are the observed data consistent with the proposed mechanism? Motivate!
c Under the experimental conditions given in Table do you expect that the
surface mainly consists of vacant sites, sites with adsorbed sites with
adsorbed or sites with adsorbed Simplify the reaction rate expression
found under a based on observations Table P and combining constants
into new ones?
d If the mechanism holds, what reaction rate do you predict for bar,
bar and bar?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


