Question: Please answer the question fully and write legibly + Problem 2 (30 points) A feed F is split into a vapor product V and a
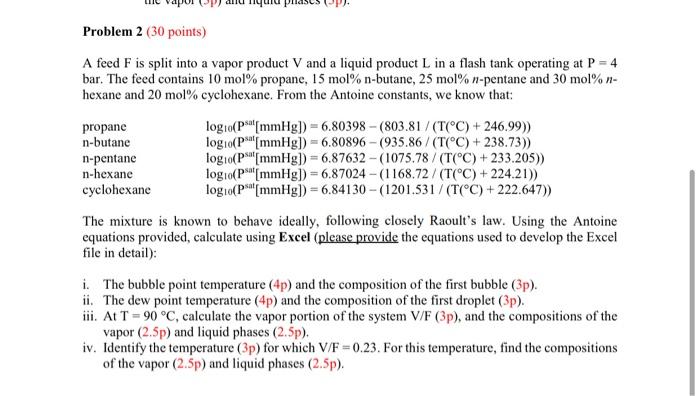
+ Problem 2 (30 points) A feed F is split into a vapor product V and a liquid product L in a flash tank operating at P = 4 bar. The feed contains 10 mol% propane, 15 mol% n-butane, 25 mol% n-pentane and 30 mol%- hexane and 20 mol% cyclohexane. From the Antoine constants, we know that: propane logo(Ps[mmHg]) = 6.80398 - (803.81/(T(C) + 246.99)) n-butane logo(pa[mmHg]) = 6.80896 - (935.86/(T(C) + 238.73)) n-pentane log 10(Ps[mmHg] =6.87632 - (1075.78 / (T(C) +233.205)) n-hexane log 10(psat[mmHg) = 6.87024 - (1168.72 / (T(C) + 224.21)) cyclohexane logo(Ps[mmHg]) = 6.84130 - (1201.531/(T(C)+222.647)) The mixture is known to behave ideally, following closely Raoult's law. Using the Antoine equations provided, calculate using Excel (please provide the equations used to develop the Excel file in detail): i. The bubble point temperature (4p) and the composition of the first bubble (3p). ii. The dew point temperature (4p) and the composition of the first droplet (3p). iii. At T = 90C, calculate the vapor portion of the system V/F (3p), and the compositions of the vapor (2.5p) and liquid phases (2.5p). iv. Identify the temperature (3p) for which V/F =0.23. For this temperature, find the compositions of the vapor (2.5p) and liquid phases (2.5p)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


