Question: please answer the questions by looking at the values provided on my table, and these values for R from the van der Waals equation. (for

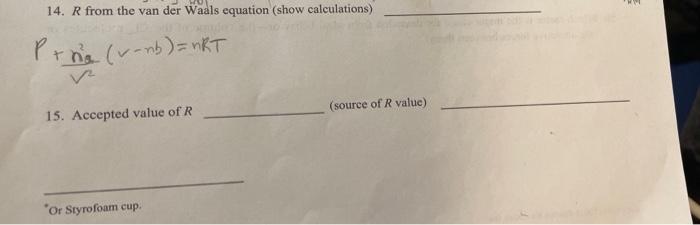


8 8 Determination of R: The Gas Law Constant 1. Mass of test tube +KCIO, + MnO 2. Mass of test tube + contents after reaction 9.870_8 3. Mass of oxygen produced 9.761 0109 4. Mass of 125 ml flask* + water 175.875 5. Mass of 125 ml flask 91.2248 6. Mass of water 84.651 B 7. Temperature of water 27C 8. Density of water og965 12 g/mL 9. Volume of water 84.047 volume of O, gus 84_947 ml 10. Barometric pressure 11. Vapor pressure of water 26.7 12. Pressure of O, gas (show calculations) 7523 marty 779 mutty - 26.7 mm Hg = 752.3 mm ty = Po = Protal-Puster 13. Gas law constant, R, from ideal gas law (show calculations) 0.083atmeal k 752.3 mmigration T: 273+27= 300k PV = nRT im: 760 mg K.PV = 0.9940.08SL = 0.083 0.0034 x 300K mw. Os 0.109 -0.0034 0 patmas2 3 = '0.99 760 mutta 32.9 der Waals equation (show calculations) 779 months T molk 14. R from the van der Waals equation (show calculations) Prhe (verb) = nRT (source of R value) 15. Accepted value of R "Or Styrofoam cup Determination of R: The Gas Law Constant 16. Uncertainty in R (show calculations) to oitsnimisia 8 8 Determination of R: The Gas Law Constant 1. Mass of test tube +KCIO, + MnO 2. Mass of test tube + contents after reaction 9.870_8 3. Mass of oxygen produced 9.761 0109 4. Mass of 125 ml flask* + water 175.875 5. Mass of 125 ml flask 91.2248 6. Mass of water 84.651 B 7. Temperature of water 27C 8. Density of water og965 12 g/mL 9. Volume of water 84.047 volume of O, gus 84_947 ml 10. Barometric pressure 11. Vapor pressure of water 26.7 12. Pressure of O, gas (show calculations) 7523 marty 779 mutty - 26.7 mm Hg = 752.3 mm ty = Po = Protal-Puster 13. Gas law constant, R, from ideal gas law (show calculations) 0.083atmeal k 752.3 mmigration T: 273+27= 300k PV = nRT im: 760 mg K.PV = 0.9940.08SL = 0.083 0.0034 x 300K mw. Os 0.109 -0.0034 0 patmas2 3 = '0.99 760 mutta 32.9 der Waals equation (show calculations) 779 months T molk
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


