Question: Please answer them correctly as i am almost out questions thank you!!! Core Chemistry Skill: Using Molar Mass as a Conversion Factor Learning Goal: To
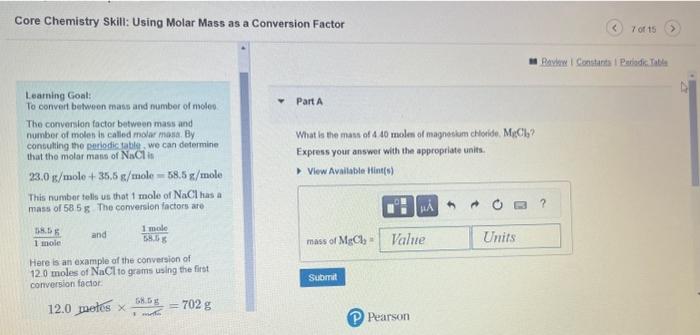
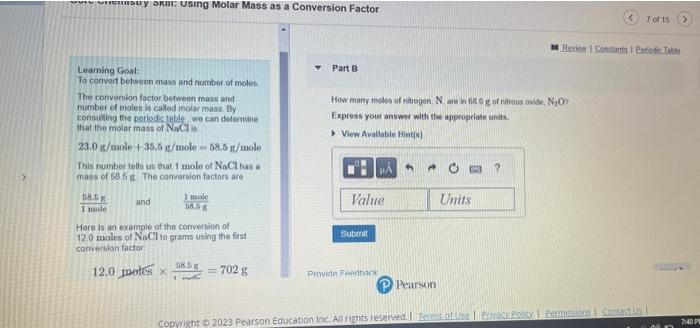
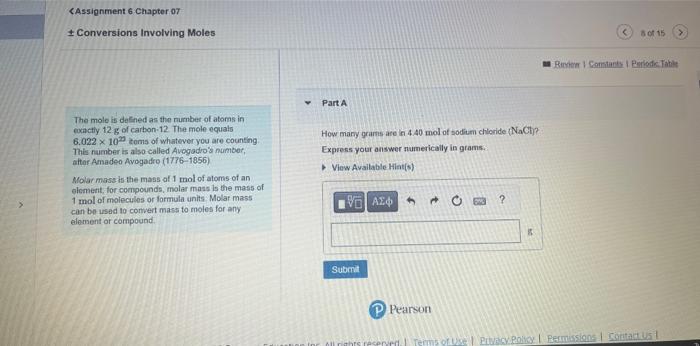
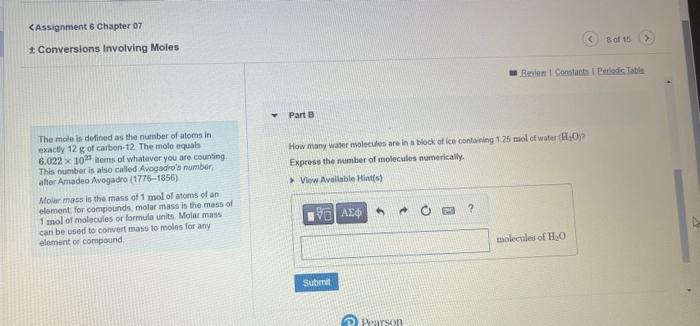
Core Chemistry Skill: Using Molar Mass as a Conversion Factor Learning Goal: To convert between mass and number of noleo Part A The conversion factor between mass and number of moles is called molar masia. By. What is the mass of 4.40 molen of magneskm chlonide. Mr icla? conbulting the periodictable, we can determine that the molar mass of NoCl is Express your answor with the appropriate units. 23.0g/mole+35.5g/mole=58.5g/ mole This number tells us that 1 mole of NaCl has a mass of 5858. The cenversion factors are. 1mole78.5F and 5.6.6g1mole Here is an example of the conversion of 12.0 moles of NaCl to grams using the firt comversion foctor: 12.0 metes 1.mti68.5g=702g (P) Pearson Loarning Goal: Part 8 Ta convert belween mass and number of moles The conversion factor between mats and number of moles is called molar mass. By How many moles of niltegon, N are n ede g of carous axde, N2O ? corisulaing the poriodc table we can detemine Express your answer with the appropriate units. that the motar mass of NaCl is 23.0g/mole+35.5g/mole=58.5gt/mole This number tells us that 1 mole of NaClhas a mass of 58.6 if The conversion factons are 1moleE8.tin and 3.5E1mole Here is an example of the conversion of 12.0 moles of NaCl to grams using the first convesion factor: 12.0 pates 1more68.5g=702g Provian Fpedhank CAssignment 6 Chapter of Conversions Involving Moles Part A The molo is de Ened as the number of atoms in comattly 12g of carbon:12. The mole equals 6.0221020 cems of whatever you are counting. How many grams are in 4.40mol of sodium chloride ( NaCl ? This number is also called Avegadro's number, Express your answer numerically in grams: atter Amadeo Avogadro (1776-1856) Molar mass is the mass of 1mol of atoms of an element, for compounds, molar mass is the mass of 1 mol of moiecules or formula units. Molar mass can be usad to convert mass to moles for any element or compound. Assignment 6 Chapter 07 Conversions Involving Moles an Revina t Constacta Part B The male in delined as the number of atoms in exactly 12g of caubon-12. The molo equals How many waser molecules are in a biock of ice containing 125mol of water H2Op. 6.0221023 lems of whatever you are counting Express the number of moleculos numerically. This number is also called Avogadro'y number: aller Amadeo Avogadro (1776-1856) Molar moss is the mass of 1mol ol atems of an element for compounds, molar mass is the mass of 1mol of molecules or formula units. Molat mass can be used to convert mass to moles for any elemerit or compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


