Question: Please answer these questions QI : Let's consider a situation where there is a device consisting of a piston and a cylinder. Initially, the cylinder
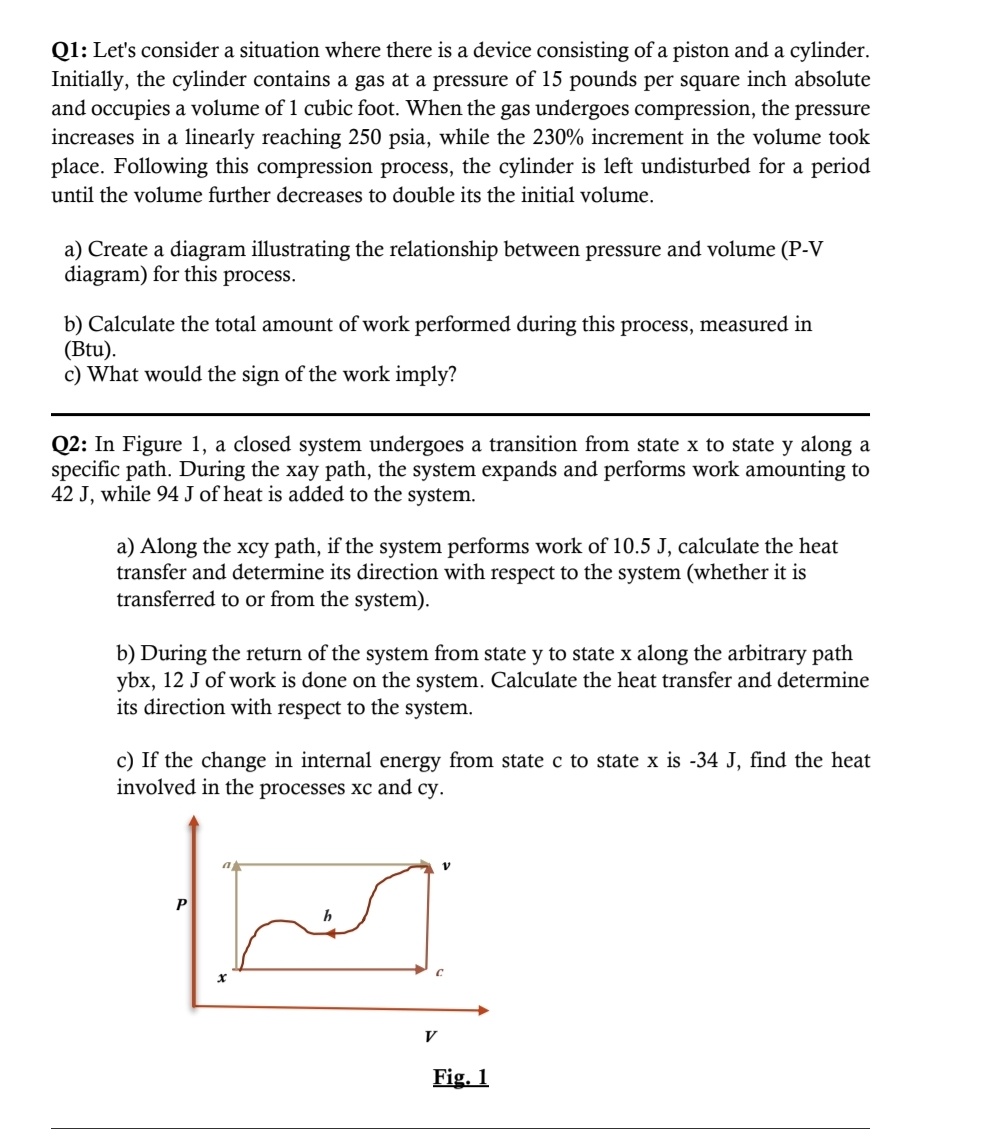
QI : Let's consider a situation where there is a device consisting of a piston and a cylinder. Initially, the cylinder contains a gas at a pressure of 15 pounds per square inch absolute and occupies a volume of 1 cubic foot. When the gas undergoes compression, the pressure increases in a linearly reaching 250 psia, while the 230% increment in the volume took place. Following this compression process, the cylinder is left undisturbed for a period until the volume further decreases to double its the initial volume. a) Create a diagram illustrating the relationship between pressure and volume (P-V diagram) for this process. b) Calculate the total amount of work performed during this process, measured in (Btu). c) What would the sign of the work imply? Q2: In Figure 1, a closed system undergoes a transition from state x to state y along a specific path. During the xay path, the system expands and performs work amounting to 42 J, while 94 J of heat is added to the system. a) Along the xcy path, if the system performs work of 10.5 J, calculate the heat transfer and determine its direction with respect to the system (whether it is transferred to or from the system). b) During the return of the system from state y to state x along the arbitrary path ybx, 12 J of work is done on the system. Calculate the heat transfer and determine its direction with respect to the system. c) If the change in internal energy from state c to state x is -34 J, find the heat involved in the processes xc and cy. h Eig.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


