Question: Please answer these two. thank you. They are two seperate questions. the graph goes with the values directly below it and the question on the
Please answer these two. thank you.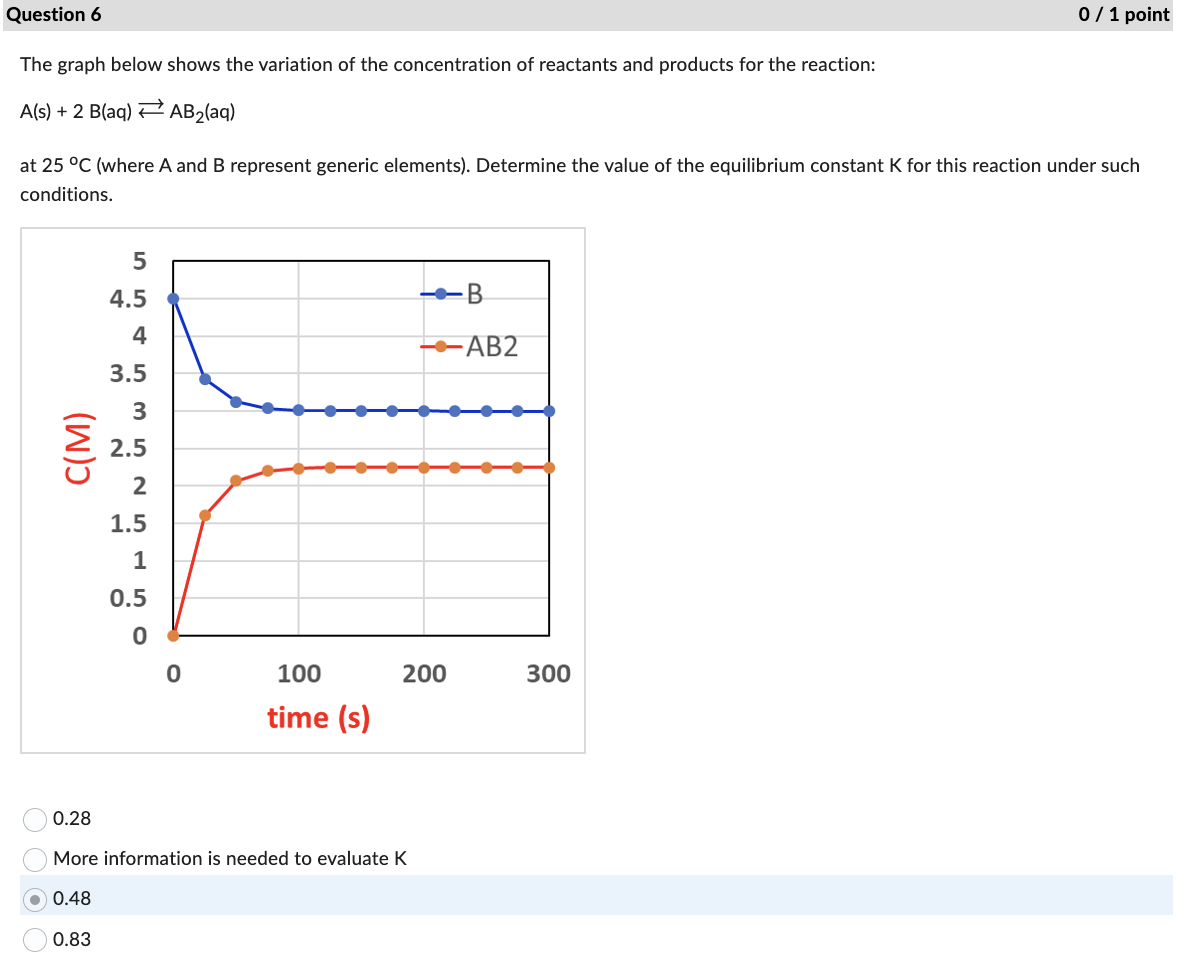

The graph below shows the variation of the concentration of reactants and products for the reaction: A(s)+2B(aq)AB2(aq) at 25C (where A and B represent generic elements). Determine the value of the equilibrium constant K for this reaction under such conditions. 0.28 More information is needed to evaluate K 0.48 0.83 Determine how much faster the reaction will be if [A] is tripled and [B] is reduced by 1/2) and the rate law =373[A]1[B]2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


