Question: please answer this complete on page by handwriting Buffer 100 um thick 20 elements 40 um thick 8 elements UO, kernel 425 um diameter 50
please answer this complete on page by handwriting
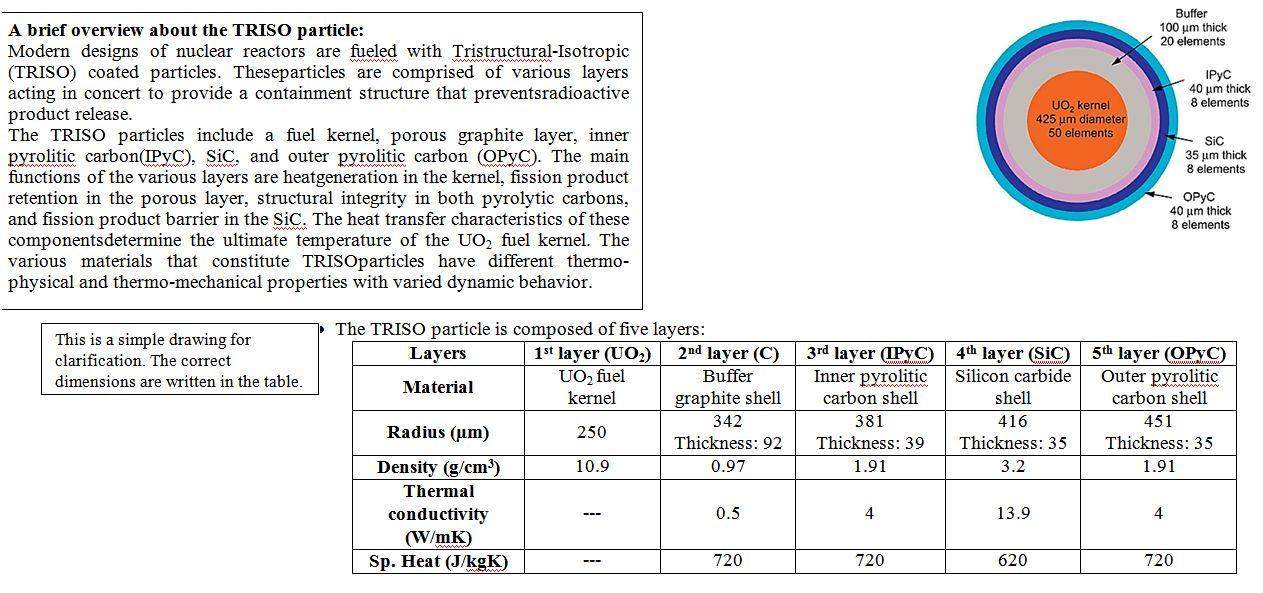
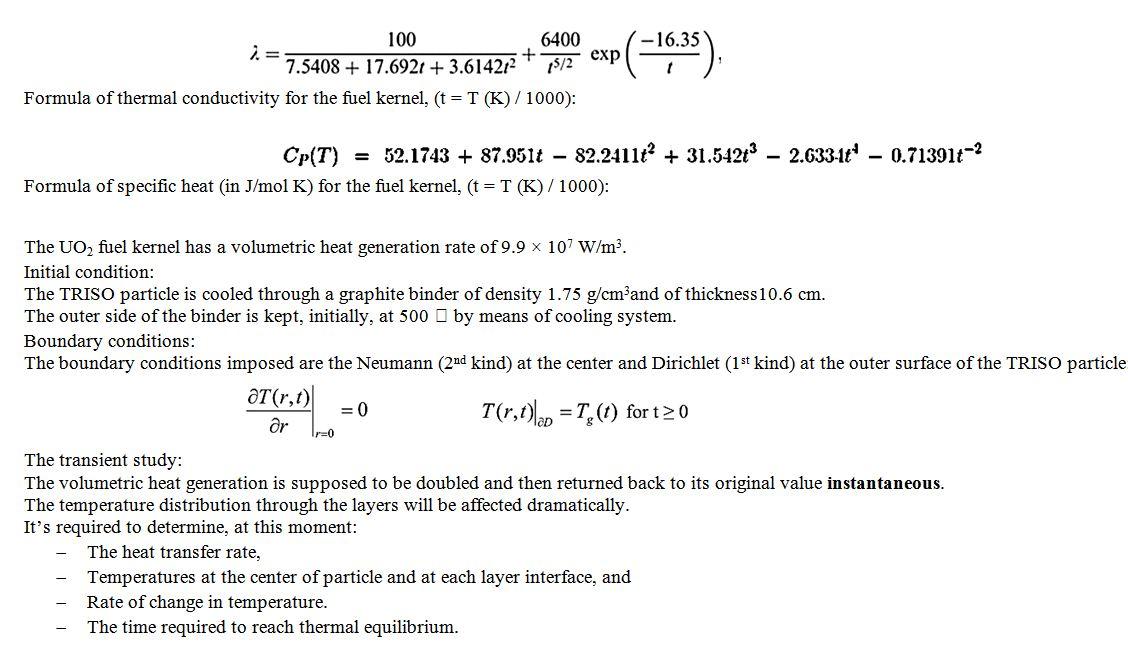
Buffer 100 um thick 20 elements 40 um thick 8 elements UO, kernel 425 um diameter 50 elements A brief overview about the TRISO particle: Modern designs of nuclear reactors are fueled with Tristructural-Isotropic (TRISO) coated particles. Theseparticles are comprised of various layers acting in concert to provide a containment structure that preventsradioactive product release. The TRISO particles include a fuel kernel, porous graphite layer, inner pyrolitic carbon(IPyC), Sic, and outer pyrolitic carbon (OPYC). The main functions of the various layers are heatgeneration in the kernel, fission product retention in the porous layer, structural integrity in both pyrolytic carbons, and fission product barrier in the Sic. The heat transfer characteristics of these componentsdetermine the ultimate temperature of the UO2 fuel kernel. The various materials that constitute TRISOparticles have different thermo- physical and thermo-mechanical properties with varied dynamic behavior. SIC 35 um thick 8 elements OPYC 40 um thick 8 elements This is a simple drawing for clarification. The correct dimensions are written in the table The TRISO particle is composed of five layers: Layers 1st layer (UO) 2nd layer (C) Buffer UO2 fuel Material kernel graphite shell 342 Radius (um) Thickness: 92 Density (g/cm) 10.9 0.97 Thermal conductivity 0.5 (W/mK) Sp. Heat (J/kgK) 720 3rd layer (LPyC) Inner pyrolitic carbon shell 381 Thickness: 39 1.91 4th layer (SIC) Silicon carbide shell 416 Thickness: 35 3.2 5th layer (OPC) Outer pyrolitic carbon shell 451 Thickness: 35 1.91 250 4 13.9 4 720 620 720 100 6400 2.= + 7.5408 + 17.6921 + 3.614212 Formula of thermal conductivity for the fuel kernel, (t = T (K)/1000): 15/2 exp (-16:35) 0.713916-2 Cp(T) = 52.1743 + 87.951t - 82.2411? + 31.542t 2.633-164 Formula of specific heat in J/mol K) for the fuel kernel, (t = T (K)/1000): The UO, fuel kernel has a volumetric heat generation rate of 9.9 x 107 W/m. Initial condition: The TRISO particle is cooled through a graphite binder of density 1.75 g/cm3and of thickness 10.6 cm. The outer side of the binder is kept, initially, at 500 by means of cooling system. Boundary conditions: The boundary conditions imposed are the Neumann (2nd kind) at the center and Dirichlet (1st kind) at the outer surface of the TRISO particle at(r,t) = 0 T(r,t)lap = T (t) for t20 or Ir=0 The transient study: The volumetric heat generation is supposed to be doubled and then returned back to its original value instantaneous. The temperature distribution through the layers will be affected dramatically. It's required to determine, at this moment: The heat transfer rate, Temperatures at the center of particle and at each layer interface, and Rate of change in temperature. The time required to reach thermal equilibrium. Buffer 100 um thick 20 elements 40 um thick 8 elements UO, kernel 425 um diameter 50 elements A brief overview about the TRISO particle: Modern designs of nuclear reactors are fueled with Tristructural-Isotropic (TRISO) coated particles. Theseparticles are comprised of various layers acting in concert to provide a containment structure that preventsradioactive product release. The TRISO particles include a fuel kernel, porous graphite layer, inner pyrolitic carbon(IPyC), Sic, and outer pyrolitic carbon (OPYC). The main functions of the various layers are heatgeneration in the kernel, fission product retention in the porous layer, structural integrity in both pyrolytic carbons, and fission product barrier in the Sic. The heat transfer characteristics of these componentsdetermine the ultimate temperature of the UO2 fuel kernel. The various materials that constitute TRISOparticles have different thermo- physical and thermo-mechanical properties with varied dynamic behavior. SIC 35 um thick 8 elements OPYC 40 um thick 8 elements This is a simple drawing for clarification. The correct dimensions are written in the table The TRISO particle is composed of five layers: Layers 1st layer (UO) 2nd layer (C) Buffer UO2 fuel Material kernel graphite shell 342 Radius (um) Thickness: 92 Density (g/cm) 10.9 0.97 Thermal conductivity 0.5 (W/mK) Sp. Heat (J/kgK) 720 3rd layer (LPyC) Inner pyrolitic carbon shell 381 Thickness: 39 1.91 4th layer (SIC) Silicon carbide shell 416 Thickness: 35 3.2 5th layer (OPC) Outer pyrolitic carbon shell 451 Thickness: 35 1.91 250 4 13.9 4 720 620 720 100 6400 2.= + 7.5408 + 17.6921 + 3.614212 Formula of thermal conductivity for the fuel kernel, (t = T (K)/1000): 15/2 exp (-16:35) 0.713916-2 Cp(T) = 52.1743 + 87.951t - 82.2411? + 31.542t 2.633-164 Formula of specific heat in J/mol K) for the fuel kernel, (t = T (K)/1000): The UO, fuel kernel has a volumetric heat generation rate of 9.9 x 107 W/m. Initial condition: The TRISO particle is cooled through a graphite binder of density 1.75 g/cm3and of thickness 10.6 cm. The outer side of the binder is kept, initially, at 500 by means of cooling system. Boundary conditions: The boundary conditions imposed are the Neumann (2nd kind) at the center and Dirichlet (1st kind) at the outer surface of the TRISO particle at(r,t) = 0 T(r,t)lap = T (t) for t20 or Ir=0 The transient study: The volumetric heat generation is supposed to be doubled and then returned back to its original value instantaneous. The temperature distribution through the layers will be affected dramatically. It's required to determine, at this moment: The heat transfer rate, Temperatures at the center of particle and at each layer interface, and Rate of change in temperature. The time required to reach thermal equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


