Question: Please answer this fully and show all steps and all calculations fully and a neatly drawn graph thank u For the reversible liquid reaction at
Please answer this fully and show all steps and all calculations fully and a neatly drawn graph thank u
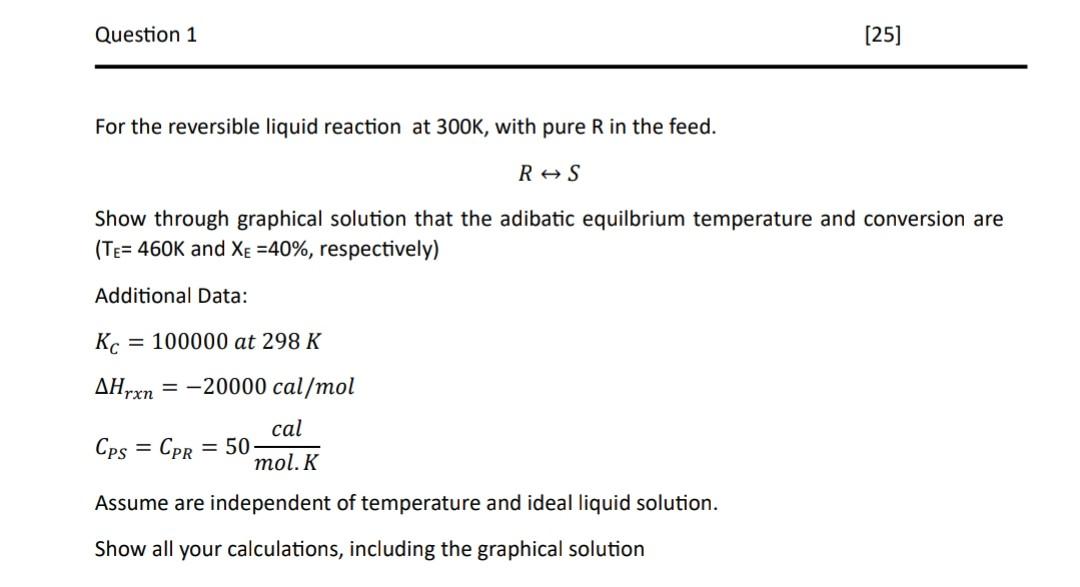
For the reversible liquid reaction at 300K, with pure R in the feed. RS Show through graphical solution that the adibatic equilbrium temperature and conversion are ( TE=460K and XE=40%, respectively) Additional Data: KC=100000at298KHrxn=20000cal/molCPS=CPR=50mol.Kcal Assume are independent of temperature and ideal liquid solution. Show all your calculations, including the graphical solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


