Question: please answer this question soon. thanks Complete the changes in concentrations (or pressure, if requested) for each of the following reactions. (a) 2SO3(g)2SO2(g)+O2(g)+x0.125M (b) 4NH3(g)+3O2(g)2N2(g)+6H2O(g)0.24M
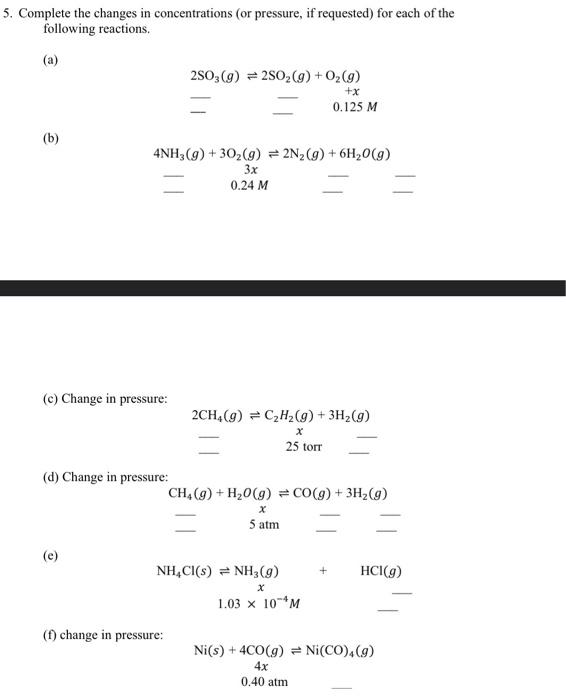
Complete the changes in concentrations (or pressure, if requested) for each of the following reactions. (a) 2SO3(g)2SO2(g)+O2(g)+x0.125M (b) 4NH3(g)+3O2(g)2N2(g)+6H2O(g)0.24M (c) Change in pressure: 2CH4(g)C2H2(g)+3H2(g)x25torr (d) Change in pressure: CH4(g)+H2O(g)CO(g)+3H2(g)x5atm (e) NH4Cl(s)NH3(g)+x+HCl(g)1.03104M (f) change in pressure: Ni(s)+4CO(g)Ni(CO)4(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


