Question: Please answer this, show your solutions in paper. I will give you a very good feedback. Thank you! In paper please :( 1. A copper
Please answer this, show your solutions in paper.I will give you a very good feedback. Thank you!In paper please :(
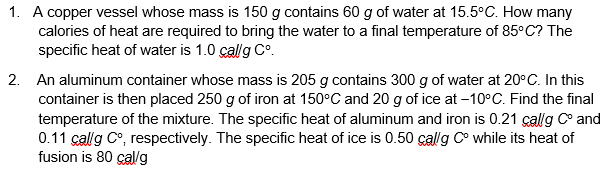
1. A copper vessel whose mass is 150 g contains 60 g of water at 15.5 C. How many calories of heat are required to bring the water to a final temperature of 85C? The specific heat of water is 1.0 cal/g Co. 2. An aluminum container whose mass is 205 g contains 300 g of water at 20*C. In this container is then placed 250 g of iron at 150C and 20 g of ice at -10 C. Find the final temperature of the mixture. The specific heat of aluminum and iron is 0.21 cal/g Co and 0.11 callg Co, respectively. The specific heat of ice is 0.50 callg Co while its heat of fusion is 80 cal/g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


