Question: Please answer this two part question. Is this aromatic? Select all that apply. Hint: Even though we have two nings here, we treat this as
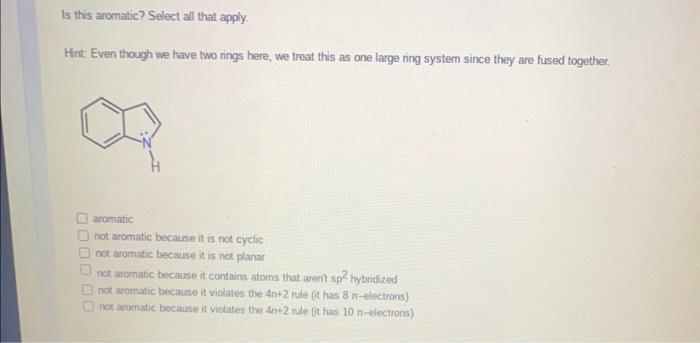
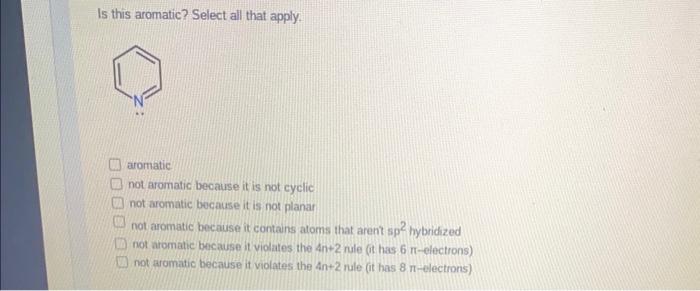
Is this aromatic? Select all that apply. Hint: Even though we have two nings here, we treat this as one large ring system since they are fused together. aromatic not aromatic because it is not cyclic not aromatic because it is not planar not aromatic because it contains atoms that arent sp 2 bybridized not aromatic because it violates the 4n+2 nile (it has 8-electrons) not aromatic because it violates the 4n+2 nule (it has 10-electrons) Is this aromatic? Select all that apply. aromatic not aromatic because it is not cyclic not aromatic because it is not planar not aromatic because it contains atoms that arent sp hybridized not aromatic because it violates the 4n+2 nule (it has 6-electrons) not aromatic because it violates the 4n+2 rule (it has 8-electrons)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


