Question: Please answer to all questions Question 1 What is the energy of a photon with wavelength 864 nm? Give your answer in units of ev
Please answer to all questions
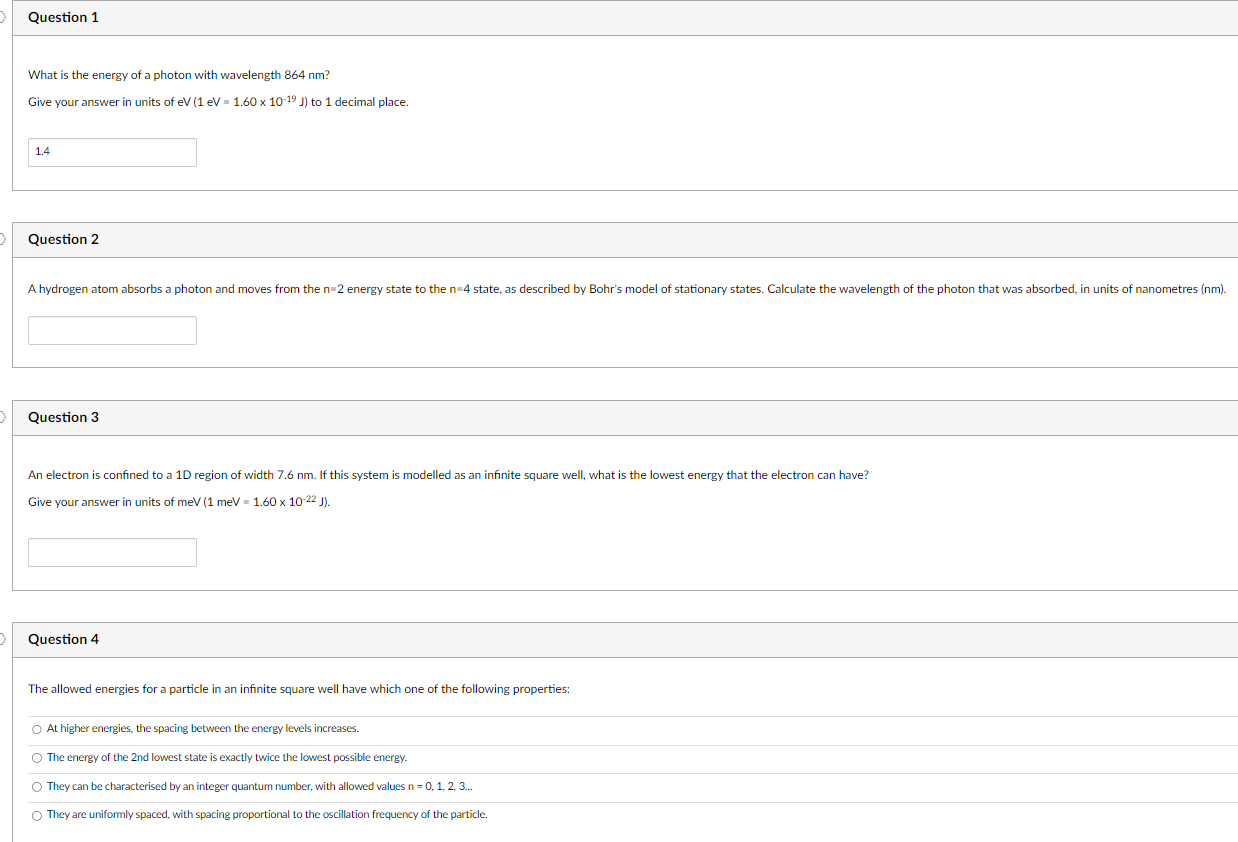
Question 1 What is the energy of a photon with wavelength 864 nm? Give your answer in units of ev (1 eV = 1.60 x 10-19 J) to 1 decimal place. 1.4 Question 2 A hydrogen atom absorbs a photon and moves from the n=2 energy state to the n=4 state, as described by Bohr's model of stationary states. Calculate the wavelength of the photon that was absorbed, in units of nanometres (nm). Question 3 An electron is confined to a 1D region of width 7.6 nm. If this system is modelled as an infinite square well, what is the lowest energy that the electron can have? Give your answer in units of meV (1 meV = 1.60 x 10-22 J). Question 4 The allowed energies for a particle in an infinite square well have which one of the following properties: At higher energies, the spacing between the energy levels increases. The energy of the 2nd lowest state is exactly twice the lowest possible energy. O They can be characterised by an integer quantum number, with allowed values n = 0, 1, 2, 3. They are uniformly spaced, with spacing proportional to the oscillation frequency of the particle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


