Question: please answer using this data 1. Make a plot of ln(AA) vs. time for each of the five kinetic runs. From the slopes of the

![From the relationship kobs=k[H]b[alcohol]c calculate the overall rate constant for each of](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d8b86d8e7_44066f8d8b80d072.jpg)
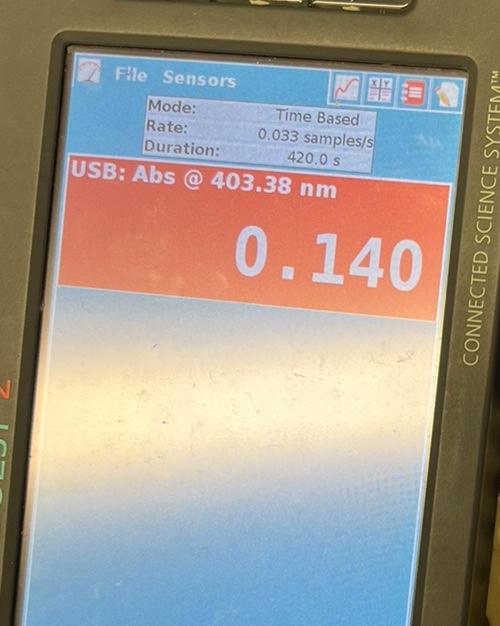
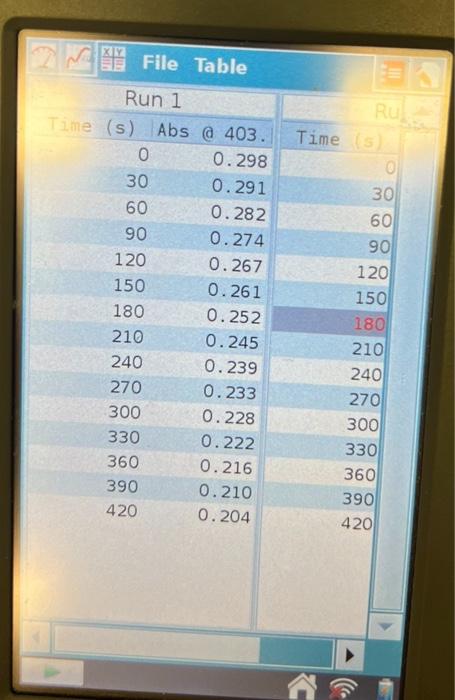
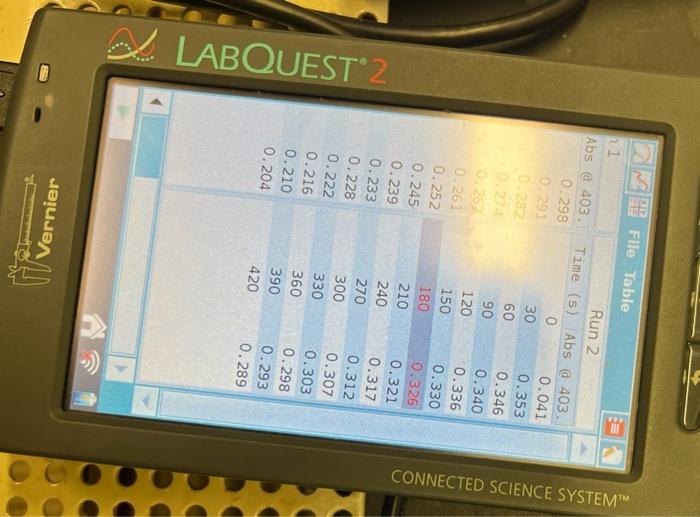
1. Make a plot of ln(AA) vs. time for each of the five kinetic runs. From the slopes of the plots determine the observed rate constant for the reaction. 2. From the relationship kobs=k[H]b[alcohol]c calculate the overall rate constant for each of the first three runs and report these values and the average value. 3. Use the values of kobs and the temperatures for paired runs two and four and three and five to determine the activation energy for the reaction. Report the individual and average values for the activation energy. Run 1 \begin{tabular}{|r|r|r|r|} \hline (s) & Abs a 403. & Time \\ \hline 0 & 0.298 & \\ \hline 30 & 0.291 & 30 \\ \hline 60 & 0.282 & 60 \\ \hline 90 & 0.274 & 90 \\ \hline 120 & 0.267 & 120 \\ 150 & 0.261 & 150 \\ \hline 180 & 0.252 & 180 \\ \hline 210 & 0.245 & 210 \\ \hline 240 & 0.239 & 240 \\ \hline 270 & 0.233 & 270 \\ \hline 300 & 0.228 & 300 \\ \hline 330 & 0.222 & 330 \\ 360 & 0.216 & 360 \\ 390 & 0.210 & 390 \\ 420 & 0.204 & 420 \\ \hline & & \\ \hline \end{tabular} 2 FHe Sensors Mode:Rate:Duration:USB:Abs@403.38nmTimeBased0.033samples/s420.0s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


