Question: please answer with clear steps (18) The reaction Fe3O4(s) + CO(g) = 3FeO(s) + CO2(g) has an equilibrium constant K = 1.15 at 600C. Find
please answer with clear steps
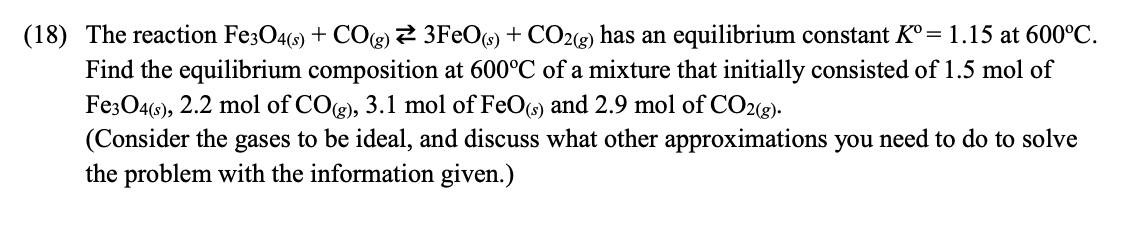
(18) The reaction Fe3O4(s) + CO(g) = 3FeO(s) + CO2(g) has an equilibrium constant K = 1.15 at 600C. Find the equilibrium composition at 600C of a mixture that initially consisted of 1.5 mol of Fe3O4(s), 2.2 mol of CO(g), 3.1 mol of FeO(s) and 2.9 mol of CO2(g). (Consider the gases to be ideal, and discuss what other approximations you need to do to solve the problem with the information given.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


