Question: PLEASE ANSWER WITH COMPLETE AND DETAILED SOLUTION 15. A solution of aqueous 10%Na2SO4 and 20%MgSO4 is crystallized in order to obtain Lweite, or Na2SO4MgSO42.5H2O. Instead
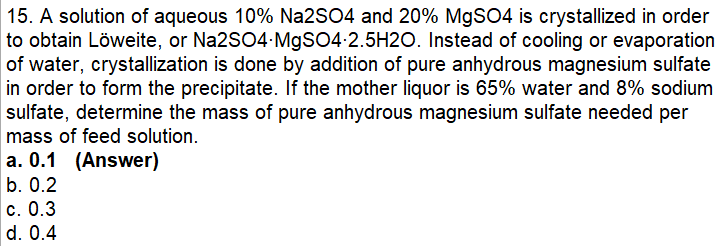 PLEASE ANSWER WITH COMPLETE AND DETAILED SOLUTION
PLEASE ANSWER WITH COMPLETE AND DETAILED SOLUTION
15. A solution of aqueous 10%Na2SO4 and 20%MgSO4 is crystallized in order to obtain Lweite, or Na2SO4MgSO42.5H2O. Instead of cooling or evaporation of water, crystallization is done by addition of pure anhydrous magnesium sulfate in order to form the precipitate. If the mother liquor is 65% water and 8% sodium sulfate, determine the mass of pure anhydrous magnesium sulfate needed per mass of feed solution. a. 0.1 (Answer) b. 0.2 c. 0.3 d. 0.4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


