Question: please answer with values in boxes as illustrated in question. unknown value in a stoichiometric reaction that involves aqueous solutions. Step 4. Related practice. The
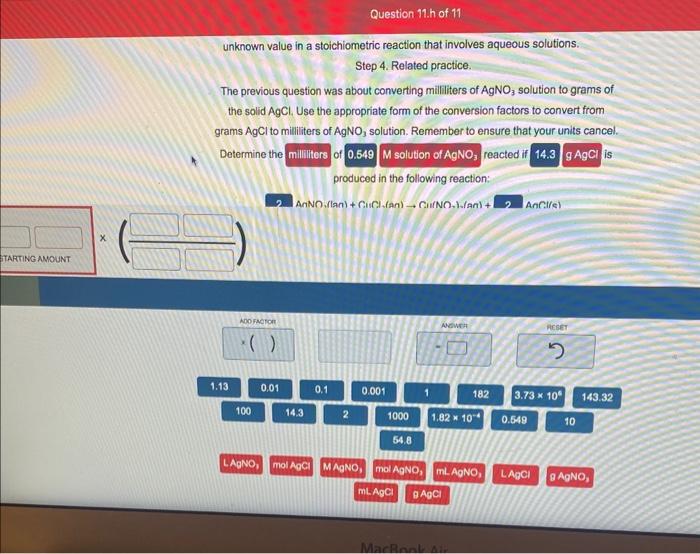

unknown value in a stoichiometric reaction that involves aqueous solutions. Step 4. Related practice. The previous question was about converting milliliters of AgNO3 solution to grams of the solid AgCl. Use the appropriate form of the conversion factors to convert from grams AgCl to milliliters of AgNO3 solution. Remember to ensure that your units cancel. Determine the of reacted if is produced in the following reaction: Step 4. Related practice. The previous question was about converting milliliters of AgNO3 solution to grams of the solid AgCl. Use the appropriate form of the conversion factors to convert from grams AgCl to milliliters of AgNO3 solution. Remember to ensure that your units cancel. Determine the milliliters of 0.549M solution of AgNO3 reacted if 14.3gAgCl is produced in the following reaction: 2AgNO3(laq)+CuCl2(aq) Cu(NO3)2(aq)+2AgCl(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


