Question: please answr all thanks! Calculations References This question has multiple parts. Work all the parts to get the most points. Describe the preparation of 330.
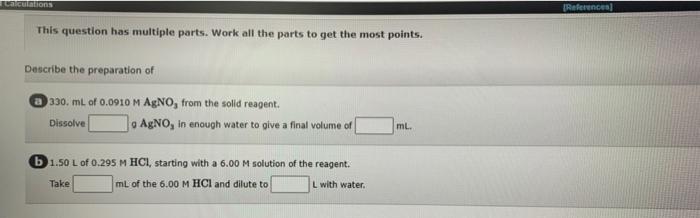
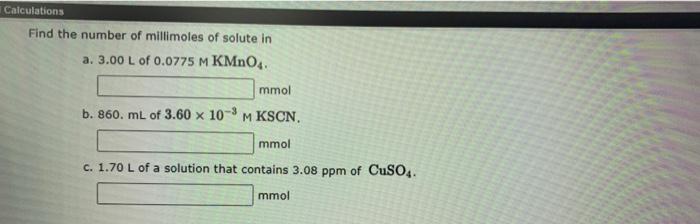
Calculations References This question has multiple parts. Work all the parts to get the most points. Describe the preparation of 330. ml. of 0.0910 M AgNO, from the solid reagent. Dissolve a AgNO, in enough water to give a final volume of g ml. b 1.50 L of 0.295 M HCI starting with a 6.00 M solution of the reagent. a Take mL of the 6.00 M HCl and dilute to L with water Calculations Find the number of millimoles of solute in a. 3.00 L of 0.0775 M KMnO4. mmol b. 860. mL of 3.60 x 10-4 M KSCN. M mmol C. 1.70 L of a solution that contains 3.08 ppm of CuSO4. mmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


