Question: Please be sure to answer otherwise don't do it The decomposition of NO in CCI at 318K has been studied by monitoring the concentration of
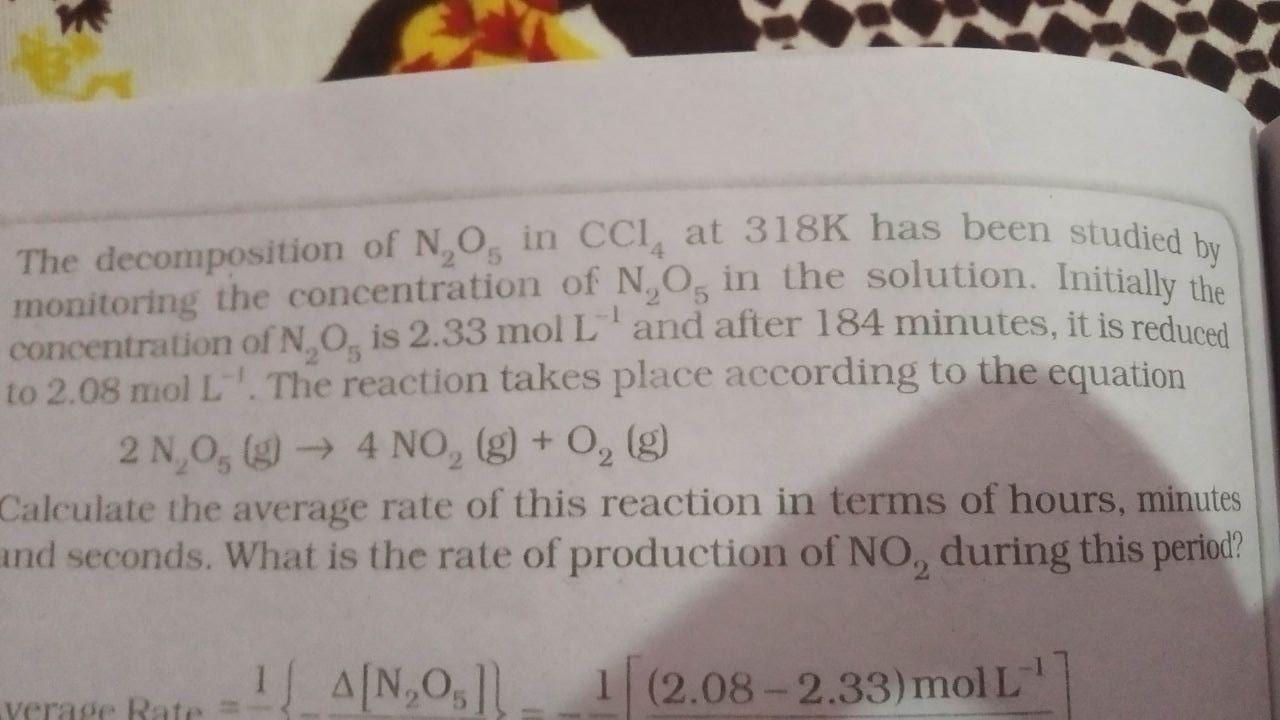 Please be sure to answer otherwise don't do it
Please be sure to answer otherwise don't do it
The decomposition of NO in CCI at 318K has been studied by monitoring the concentration of N, O, in the solution. Initially the concentration of N, O, is 2.33 mol L and after 184 minutes, it is reduced to 2.08 mol L-'. The reaction takes place according to the equation 2 1,05 (9) 4 NO, (g) + 0, (g) Calculate the average rate of this reaction in terms of hours, minutes and seconds. What is the rate of production of NO, during this period? 1 A[N,Os1 1 (2.08 -2.33)moll verage Rate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


