Question: Please can you help me with these 3 questions. Consider the following reaction: 2 CH,OHO) 2 CO(g) + 4H2(g) Kp?_at 700 K Determine the value

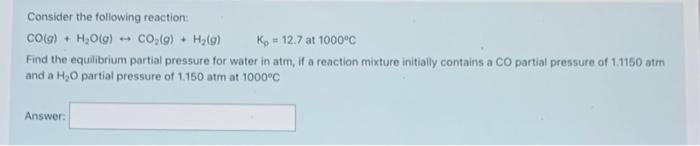
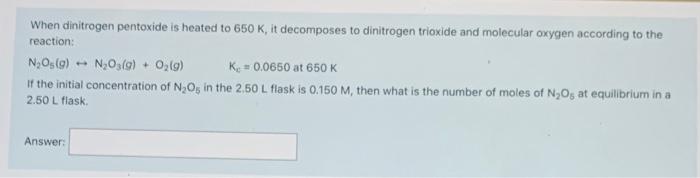
Consider the following reaction: 2 CH,OHO) 2 CO(g) + 4H2(g) Kp?_at 700 K Determine the value of Kat 700 K for the above reaction if the reaction mixture in a 0.500 L flask initially contained 0 850 atm CH,OHO). 0.080 atm cog) and 0.200 atm Hy(). The reaction was allowed to reach equilibrium, and then the partial pressure for hydrogen was determined to be 0.950 atm, Answer: Consider the following reaction: CO(g) + H2O(g) --- CO(g) + H2(0) Ky = 12.7 at 1000c Find the equilibrium partial pressure for water in atm, if a reaction mixture initially contains a CO partial pressure of 1.1150 am and a H, partial pressure of 1.150 atm at 1000C Answer: When dinitrogen pentoxide is heated to 650 K, it decomposes to dinitrogen trioxide and molecular oxygen according to the reaction: No Os(a) - N,O3(a) - 0,(9) Ke + 0.0650 at 650K If the initial concentration of N Os in the 250 L flask is 0.150 M, then what is the number of moles of N Os at equilibrium in a 2.50 L flask
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


