Question: please checj if these are correct 4.00 PM Mon Jan 25 Determine if the following statements are true or false. Ionic compounds form when ions
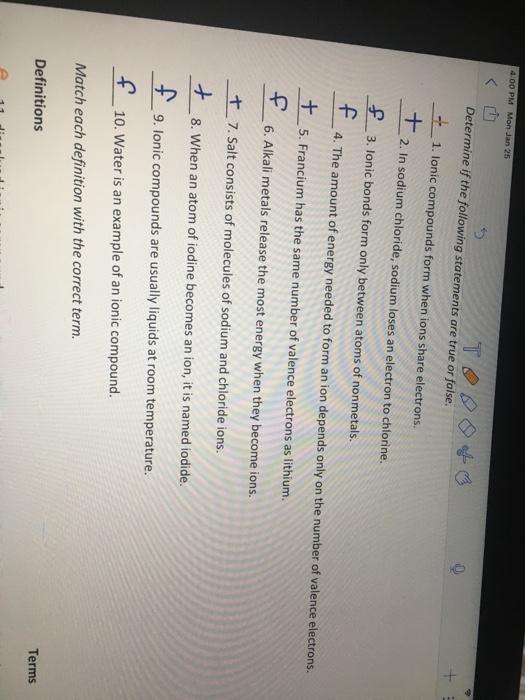

4.00 PM Mon Jan 25 Determine if the following statements are true or false. Ionic compounds form when ions share electrons. + 2. In sodium chloride, sodium loses an electron to chlorine. f 3. lonic bonds form only between atoms of nonmetals. f 4. The amount of energy needed to form an ion depends only on the number of valence electrons. + 5. Francium has the same number of valence electrons as tithium f 6. Alkali metals release the most energy when they become ions. + 7. Salt consists of molecules of sodium and chloride ions. 7 8. When an atom of iodine becomes an ion, it is named iodide. f 9. Ionic compounds are usually liquids at room temperature. f 10. Water is an example of an ionic compound. Match each definition with the correct term. Definitions Terms 25. Describe the crystal structure of ionic compounds. ridge cryst and brew and strony, tales lots of energy to break 26. List properties of ionic compounds, crystal shape, nigh melting point good conducters of electriy in liquet store 27. Explain why ionic compounds are solids at room temperature and why they cannot conduct electricity in the solid state. ger locked into plea by Strong bonds in solid state theje less freely and cannot carry an electric current
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


