Question: please correct answer Identify limiting reactants (maximum product method). Close Problern Consider the reaction of phosphorus with water and bromine. 2P(s)+6H2O(1)+3Br2(1)6HBr(aq)+2H3PO3(aq) Determine the limiting reactant
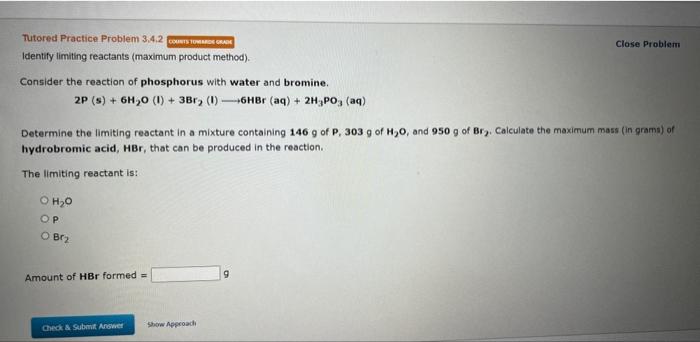
Identify limiting reactants (maximum product method). Close Problern Consider the reaction of phosphorus with water and bromine. 2P(s)+6H2O(1)+3Br2(1)6HBr(aq)+2H3PO3(aq) Determine the limiting reactant in a mixture containing 146g of P,303g of H2O, and 950g of Br. Calculate the maximum mass (in grams) of hydrobromic acid, HBr, that can be produced in the reaction. The limiting reactant is: H2O P Br2 Amount of HBr formed = 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


