Question: Please correct those answers Report Table CF.1: Rounding Measurements Table view List view Answer the following questions about values rounded by a student to three
Please correct those answers
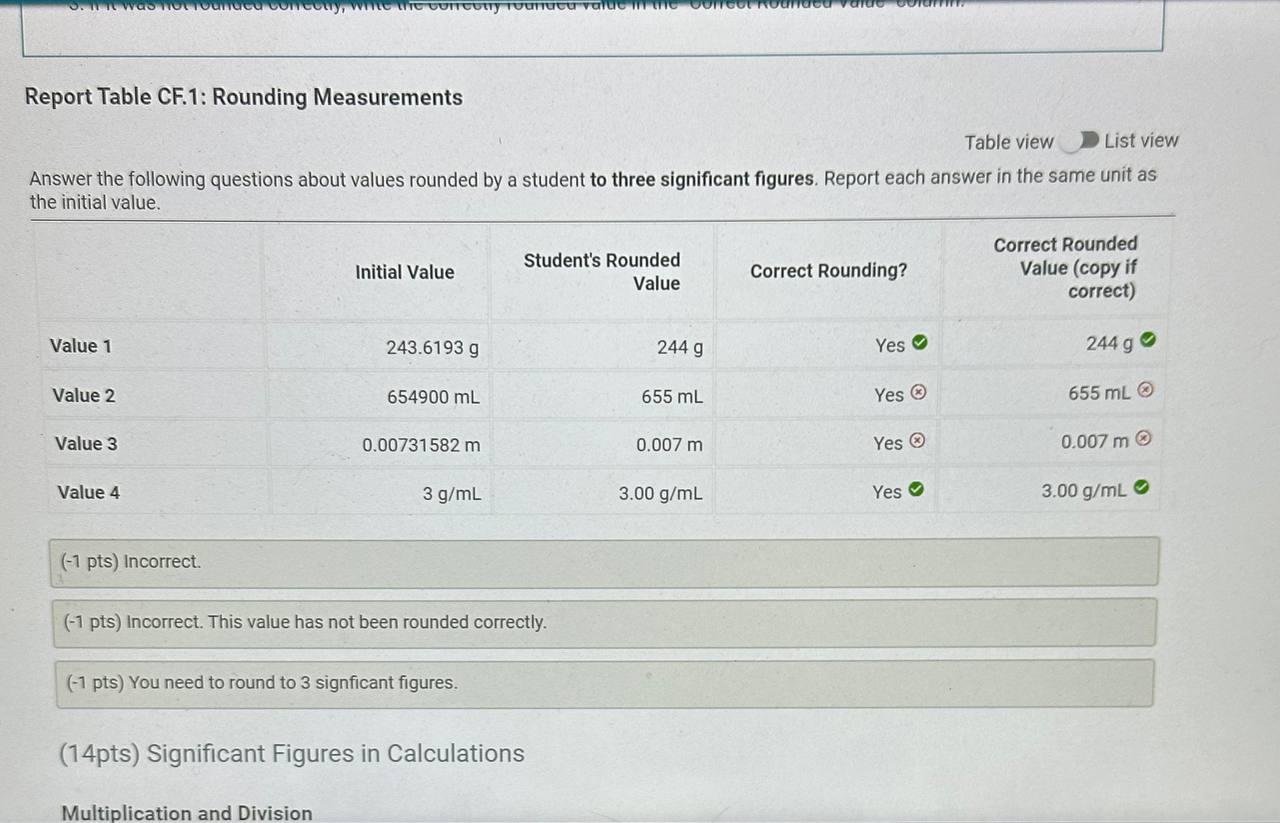
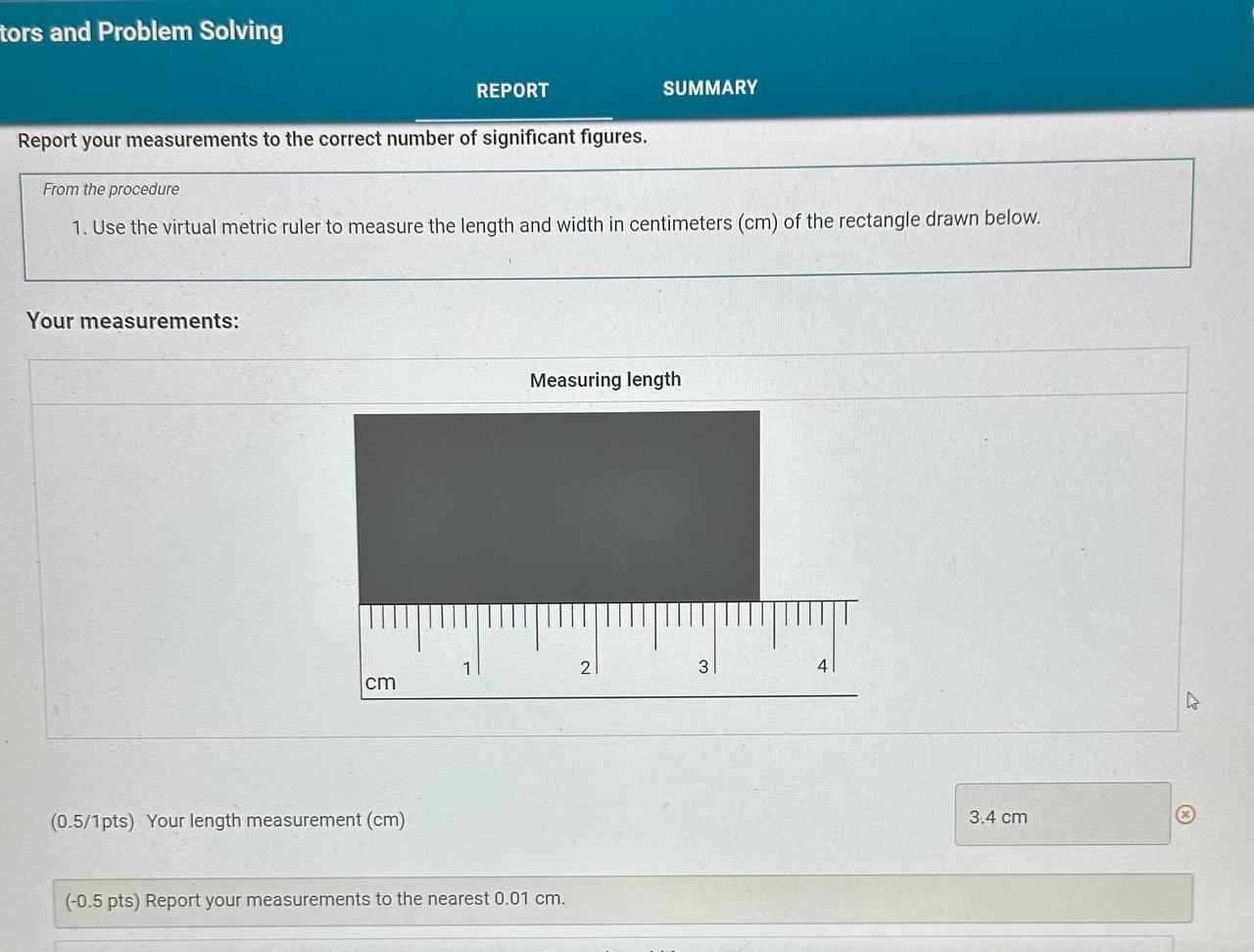
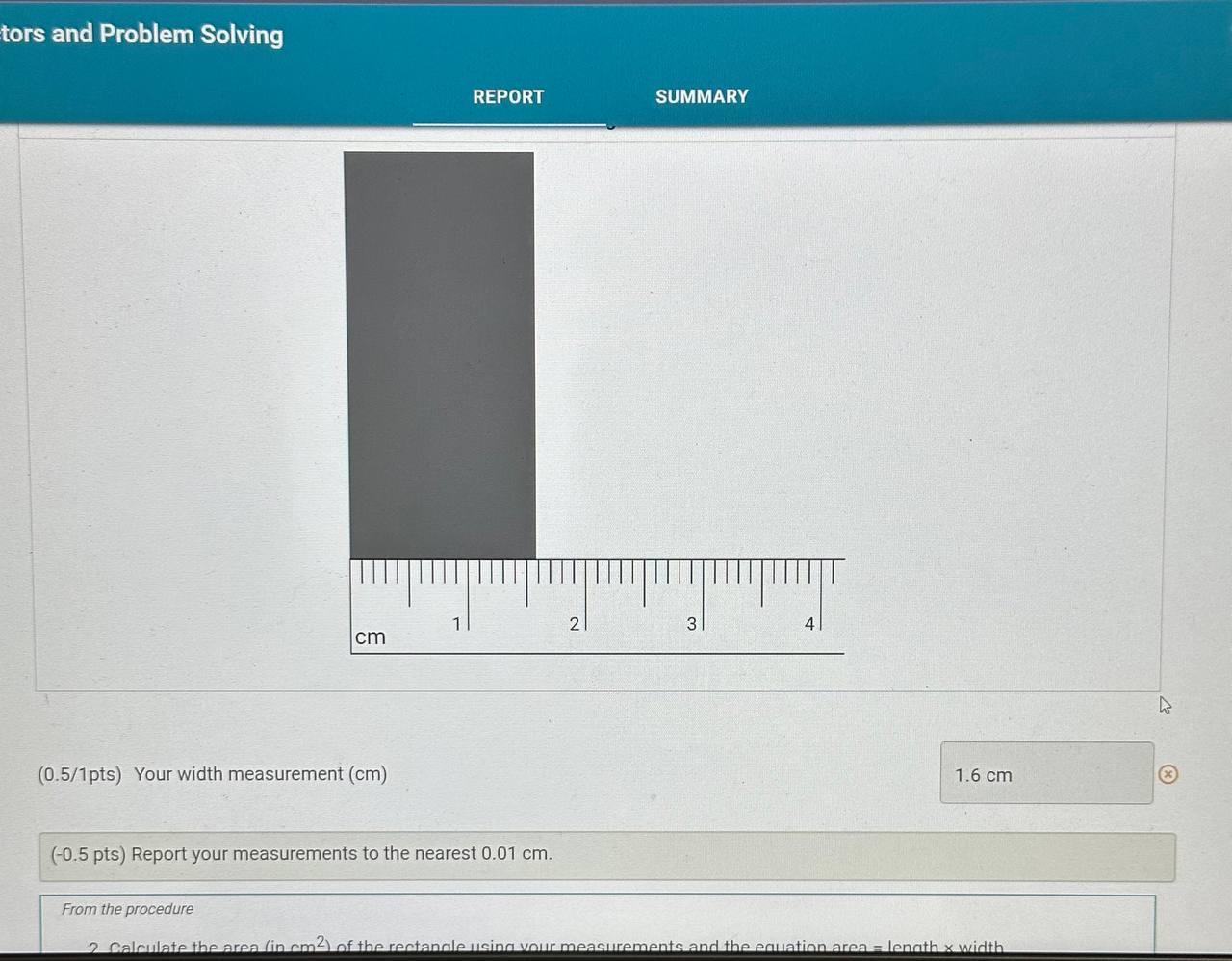
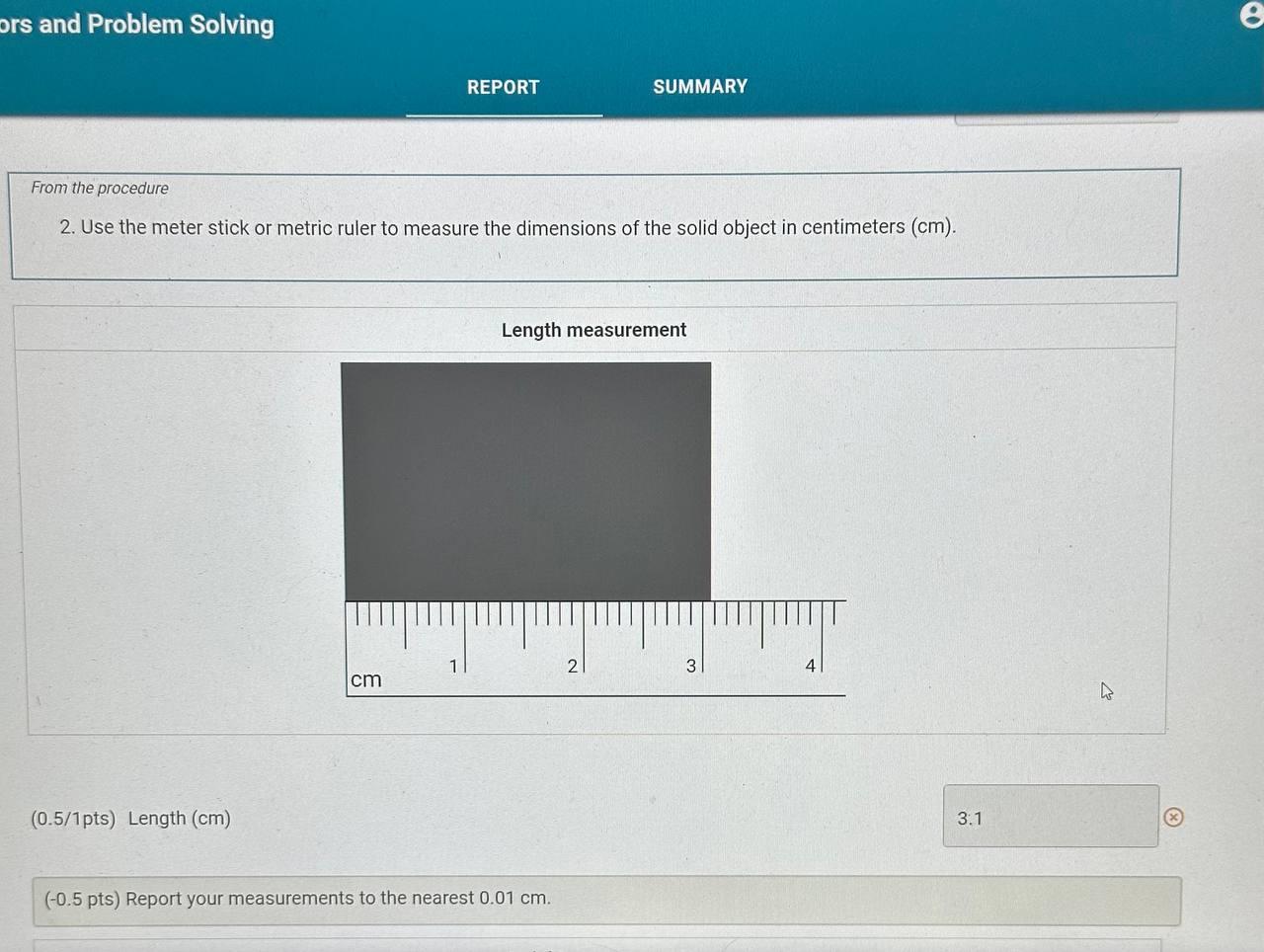
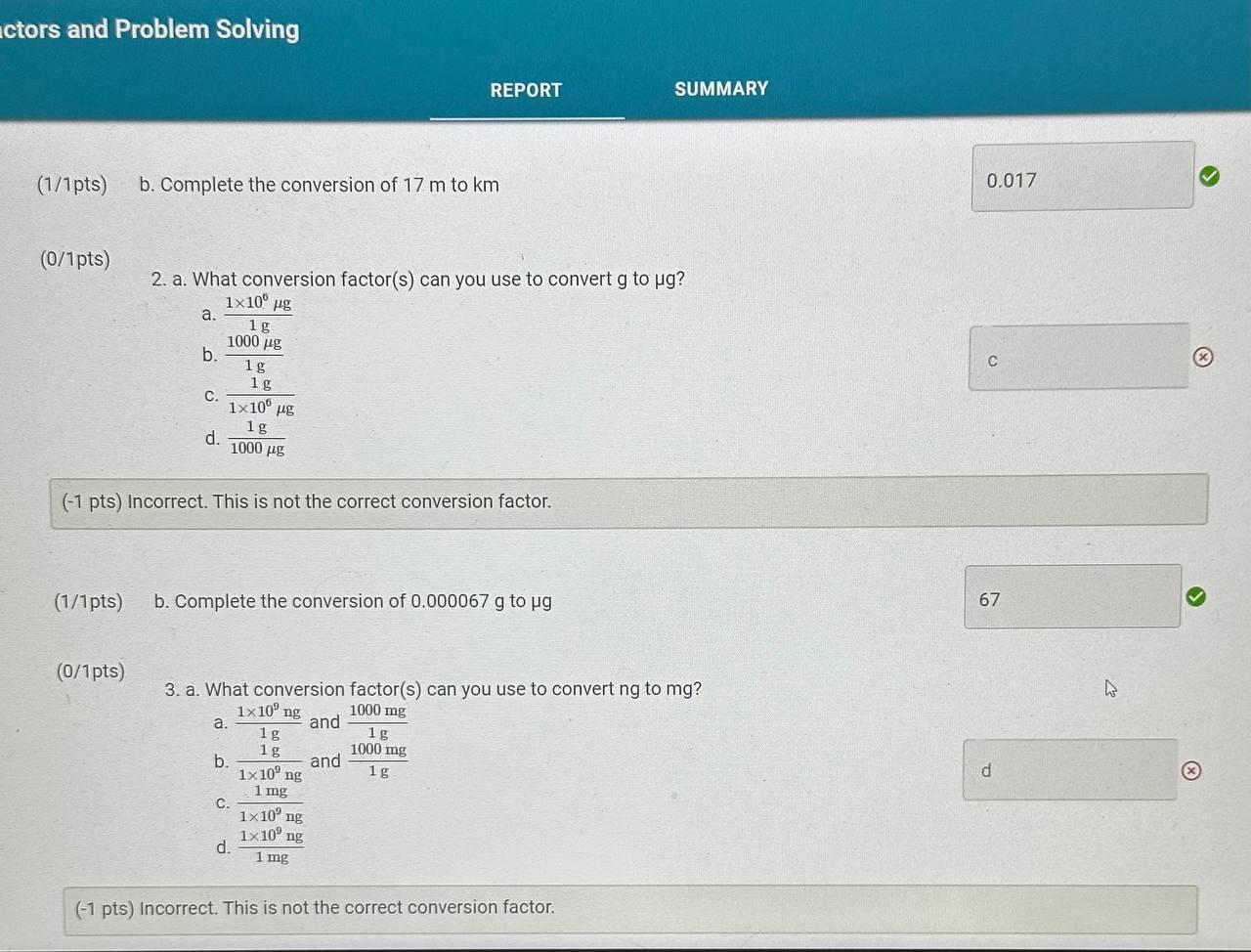
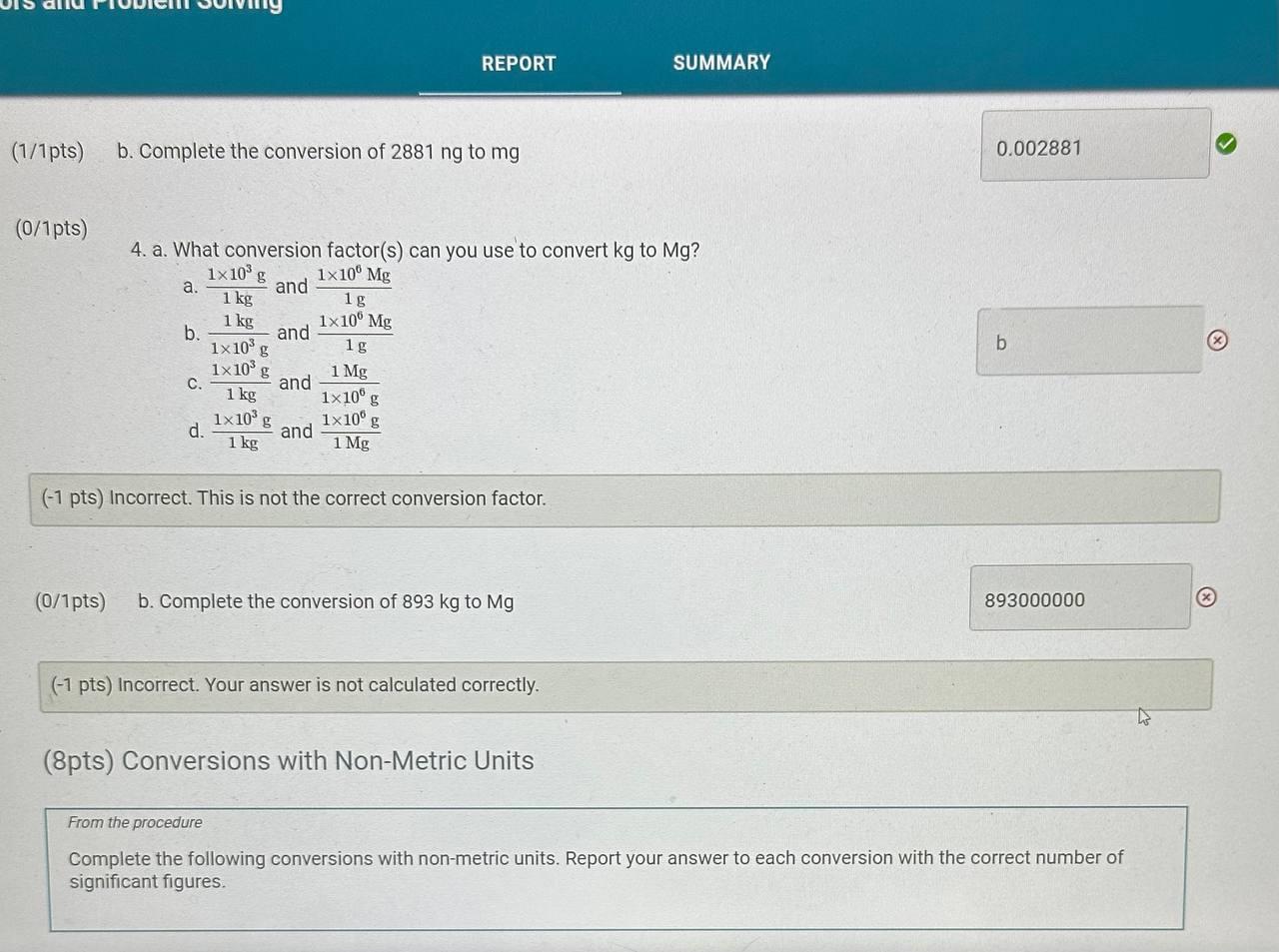
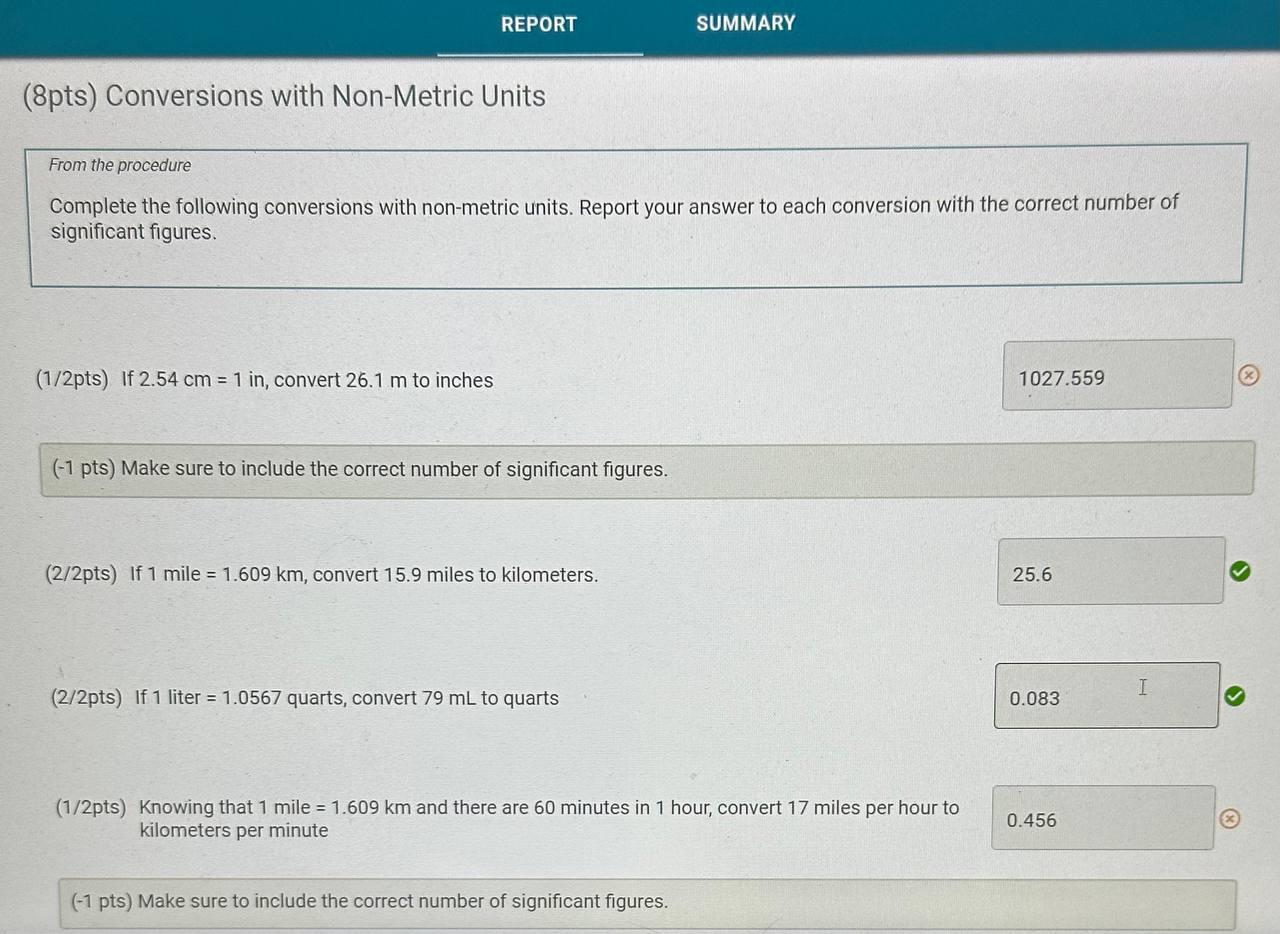
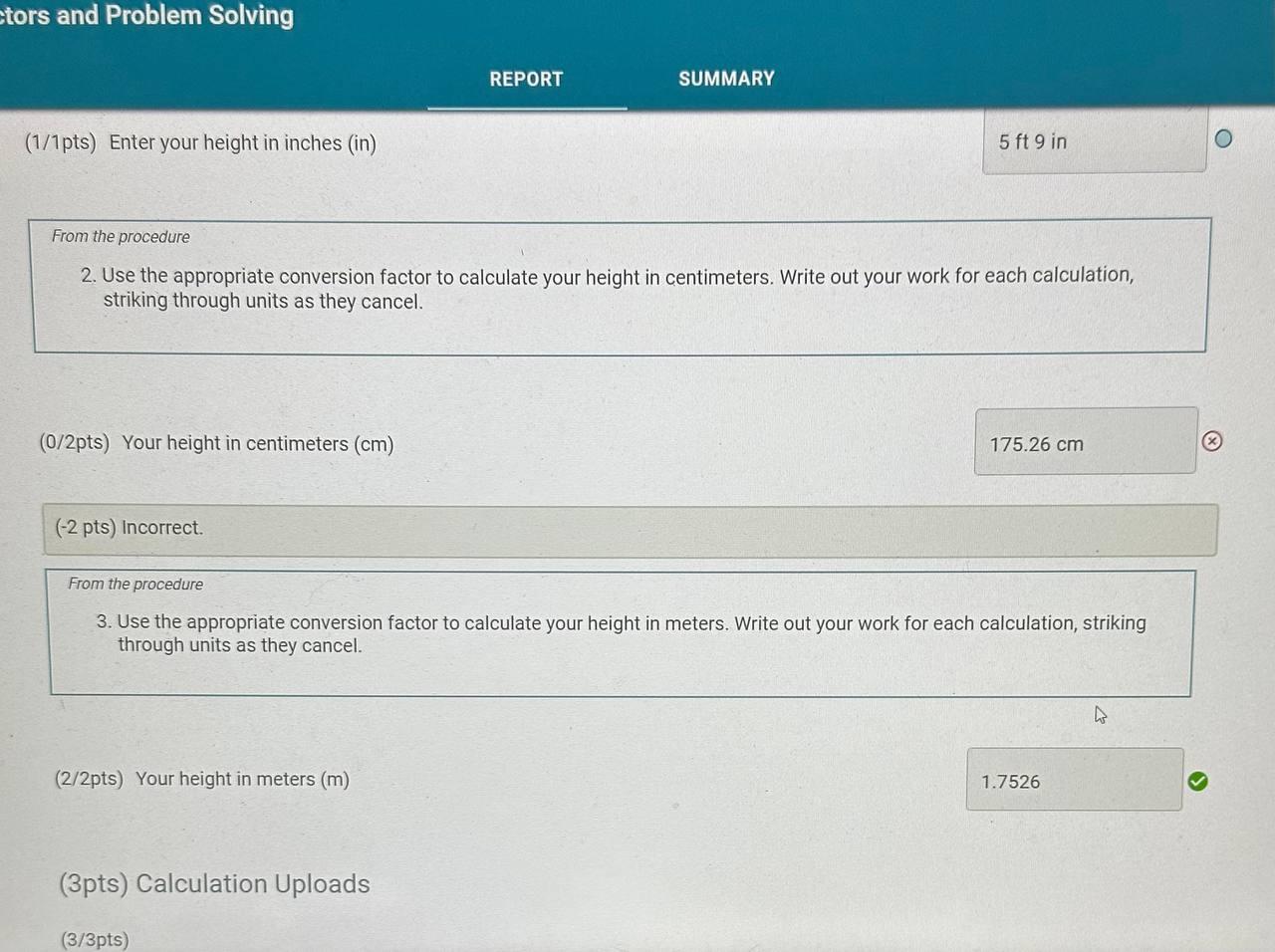
Report Table CF.1: Rounding Measurements Table view List view Answer the following questions about values rounded by a student to three significant figures. Report each answer in the same unit as the initial value. tors and Problem Solving REPORT Report your measurements to the correct number of significant figures. From the procedure 1. Use the virtual metric ruler to measure the length and width in centime Your measurements: (0.5/1pts) Your length measurement (cm) (0.5 pts) Report your measurements to the nearest 0.01cm. tors and Problem Solving (0.5/1pts) Your width measurement ( cm) (0.5 pts) Report your measurements to the nearest 0.01cm. and Problem Solving From the procedure 2. Use the meter stick or metric ruler to measure the dimensions of the solid object in centimeters (cm). Length measurement (0.5/1pts) Length (cm) ( 0.5 pts) Report your measurements to the nearest 0.01cm. ctors and Problem Solving (1/1 pts) b. Complete the conversion of 17m to km (0/1pts) 2. a. What conversion factor(s) can you use to convert g to g ? a. 1g1106g b. 1g1000g c. 1106g1g d. 1000g1g (-1 pts) Incorrect. This is not the correct conversion factor. (1/1pts) b. Complete the conversion of 0.000067g to g (0/1pts) 3. a. What conversion factor(s) can you use to convert ng to mg ? a. 1g1109ng and 1g1000mg b. 1109ng1g and 1g1000mg c. 1109ng1mg d. 1mg1109ng (1 pts) Incorrect. This is not the correct conversion factor. 4. a. What conversion factor(s) can you use to convert kg to Mg ? a. 1kg1103g and 1g1106Mg b. 1103g1kg and 1g1106Mg C. 1kg1103g and 1106g1Mg d. 1kg1103g and 1Mg1106g (-1 pts) Incorrect. This is not the correct conversion factor. (0/1pts) b. Complete the conversion of 893kg to Mg (8pts) Conversions with Non-Metric Units From the procedure Complete the following conversions with non-metric units. Report your answer to each conversion with the correct number of significant figures. (8pts) Conversions with Non-Metric Units From the procedure Complete the following conversions with non-metric units. Report your answer to each conversion with the correct number of significant figures. ( 1/2pts) If 2.54cm=1in, convert 26.1m to inches (-1 pts) Make sure to include the correct number of significant figures. (2/2pts) If 1 mile =1.609km, convert 15.9 miles to kilometers. (2/2pts) If 1 liter =1.0567 quarts, convert 79mL to quarts ( 1/2 pts) Knowing that 1 mile =1.609km and there are 60 minutes in 1 hour, convert 17 miles per hour to kilometers per minute (-1 pts) Make sure to include the correct number of significant figures. tors and Problem Solving (1/1pts) Enter your height in inches (in) 5ft9 in From the procedure 2. Use the appropriate conversion factor to calculate your height in centimeters. Write out your work for each calculation, striking through units as they cancel. (0/2pts) Your height in centimeters (cm) (-2 pts) Incorrect. From the procedure 3. Use the appropriate conversion factor to calculate your height in meters. Write out your work for each calculation, striking through units as they cancel. (2/2pts) Your height in meters (m) (3pts) Calculation Uploads
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


